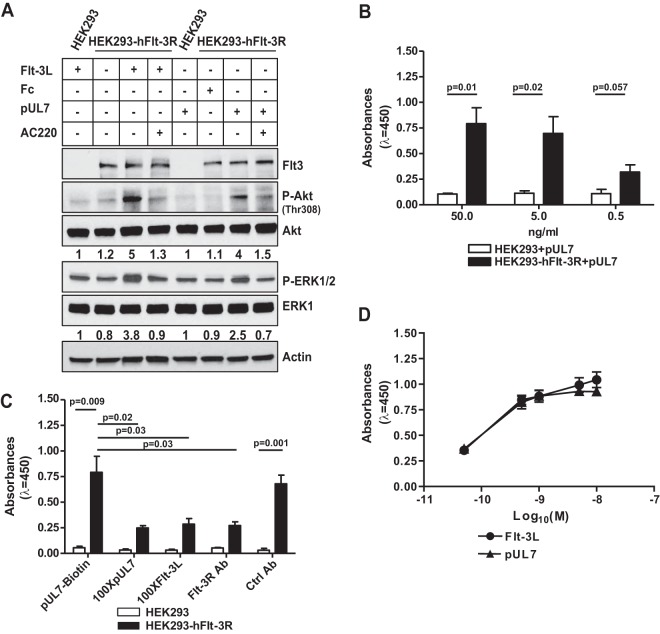
FIG 2 .
UL7 binds and signals through Flt-3R. (A) HEK-293 cells and HEK-293 cells expressing human Flt-3R (HEK-293-hFlt-3R) were serum starved, stimulated with 50 ng/ml of purified UL7 (pUL7), Fc, or Flt-3L for 10 min in the presence (+) or absence (−) of 100 nM AC220. Protein lysates were generated and immunoblotted for phosphorylation of Akt, and ERK1/2. Equal loading was confirmed by Flt-3R, Akt, ERK1, and actin antibody (Ab). Numbers under the blot indicate relative phosphorylation of Akt (Thr308) or ERK1/2 normalized to Akt or ERK1 amount and to the value of 293 cells treated with Flt-3L or Fc. Results are representative of three independent experiments. (B) HEK-293 and HEK-293-hFlt-3R cells were incubated with 50, 5, and 0.5 ng/ml of pUL7 or Fc for 1 h. The levels of pUL7 binding are expressed in absorbance units (λ = 450 nm). Values are means ± standard error of the means (SEM) (error bars) from three independent experiments. Statistical significance was determined using unpaired t test (P values are shown). (C) HEK-293 and HEK-293-hFlt-3R cells were incubated with biotinylated pUL7 (pUL7-biotin) in the presence of 100-fold excess of nonbiotinylated pUL7 (100XpUL7), or 100-fold excess Flt-3L (100XFlt-3L), or anti-Flt-3R antibody (anti-Flt-3R Ab), or a control antibody (Ctrl Ab). Values are means plus SEM (error bars) from three independent experiments. Statistical significance was determined using unpaired t test (P values are shown). (D) Serum-starved HEK-293-hFlt-3R cells were treated for 10 min with increasing concentrations of Flt-3L or pUL7 (M) (0.05 to 10 nM). Phosphorylation levels of Flt-3R were determined by PathScan phospho-Flt-3R (panTyr) ELISA. Values are means ± SEM (error bars) from three assays with each concentration measured in triplicate.