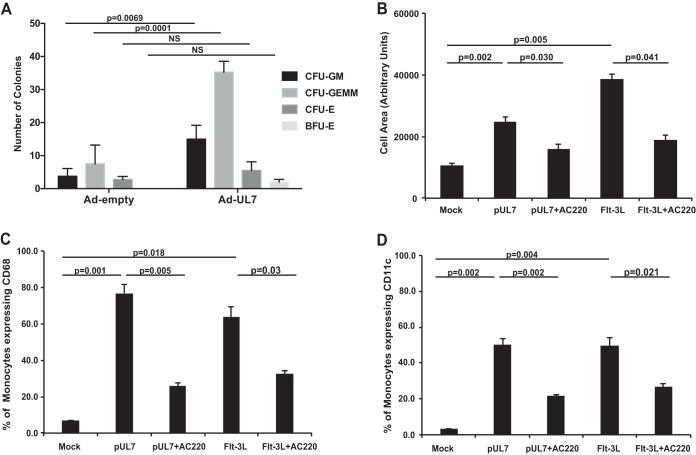
FIG 3 .
UL7 induces myelopoiesis and monocyte differentiation. (A) CD34+ HPCs were infected with adenovirus expressing UL7 (Ad-UL7) or empty adenovirus (Ad-Empty) for 24 h, sorted for viable, CD34+ HPCs, and plated in cytokine-rich methylcellulose. CFU for myeloid (granulocyte-macrophage [CFU-GM] and granulocyte-erythrocyte-monocyte-megakaryocyte progenitors [CFU-GEMM]) and erythroid (erythroid progenitors [CFU-E] and burst-forming units [BFU-E]) colonies were scored microscopically 14 days after infection. Statistical significance was determined using two-way analysis of variance followed by Sidak’s posthoc test (P values are shown; NS, not significant). (B) Peripheral blood monocytes were treated with pUL7 or Flt-3L (50 ng/ml) in the presence or absence of AC220 (100 nM), then plated on fibronectin-coated glass coverslips, and incubated for 6 days. Cells were fixed and visualized with a Leica TCS SP5 confocal microscope. The average size (in arbitrary units) of at least 50 monocytes for each experimental arm was determined from the captured images using ImageJ software. (C) Monocytes were incubated for 4 days with pUL7 or Flt-3L (50 ng/ml) in the presence or absence of AC220, then fixed, and stained with anti-CD68 Ab-conjugated to PE and analyzed by flow cytometry. (D) Monocytes were incubated for 4 days with pUL7 or Flt-3L (50 ng/ml) in the presence or absence of AC220, then fixed, and stained with anti-CD11c Ab conjugated to Alexa Fluor 700 and analyzed by flow cytometry. In each graph, values are means ± SEM (error bars) from three independent experiments using different human donors. Statistical significance was determined using one-way analysis of variance followed by Tukey’s posthoc test (P values are shown).