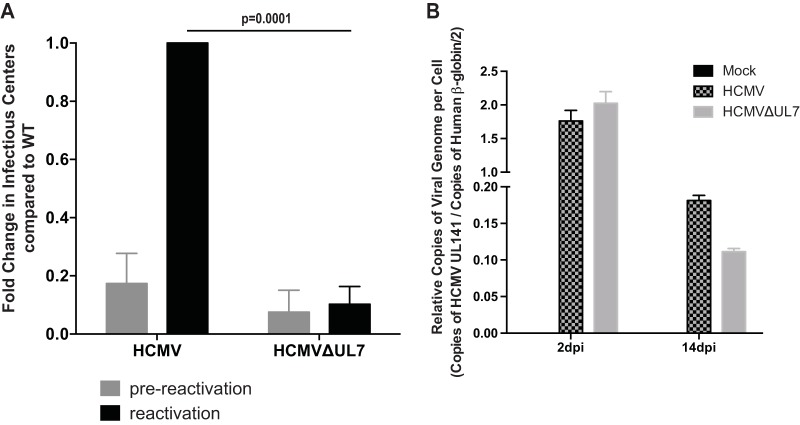
FIG 4 .
UL7 is required for HCMV reactivation in vitro. (A) Pure populations of CD34+ HPCs infected with WT HCMV or ΔUL7 mutant were isolated by FACS at 42 hpi and maintained in LTBMC medium. At 14 days postinfection (dpi), viable CD34+ HPCs were seeded onto fibroblast monolayers plated in 96-well dishes by limiting dilution in cytokine-rich medium for reactivation. An equivalent number of cells was mechanically disrupted and seeded in parallel to determine the infectious virus present in the cultures prior to reactivation (prereactivation). The frequency of infectious center formation pre- and postreactivation was determined 14 days later from the number of GFP-positive (GFP+) wells at each dilution using extreme limiting dilution analysis. The values shown are the fold change in reactivation normalized to that of WT HCMV postreactivation. Values are means plus SEM (error bars) from three independent experiments using different human donors. Statistical significance was determined using unpaired t test (P values are shown). (B) Total DNA from primary CD34+ HPC cultures was extracted at 2 dpi and 14 dpi (12 days after latency culture initiation, latency established) and HCMV genomes were quantified using quantitative PCR with primers and probe specific for the UL141 gene. Viral genomes synthesized during infection in CD34+ HPCs were normalized to the total cell number determined using human β-globin as a reference. Data shown are the mean values for three replicate quantitative PCR (qPCR), and error bars represent standard deviations. Data shown are representative of three independent experiments using different donor CD34+ HPCs. See also Fig. S3 in the supplemental material.