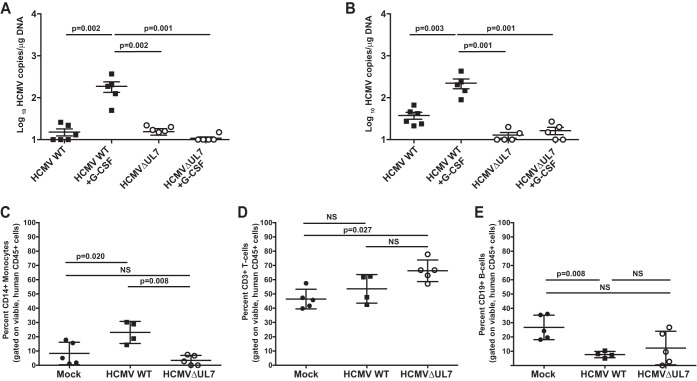
FIG 5 .
UL7 is required for HCMV reactivation and myelopoiesis in vivo. Sublethally irradiated NOD-scid IL2Rγcnull mice were engrafted with CD34+ HPCs (huNSG) and subsequently injected with human fibroblasts previously infected with WT HCMV or ΔUL7 mutant as indicated. huNSG mice injected with uninfected fibroblasts served as a negative control (n = 5). At 8 weeks postinfection, viral reactivation was triggered by treating latently infected WT HCMV and HCMVΔUL7 (n = 5) animals with G-CSF and AMD-3100. At 1 week posttreatment, mice were euthanized and tissues were harvested. Total genomic DNA was isolated from spleen (A) and liver (B) tissues, and HCMV genomes were quantified using quantitative PCR with primers and probe specific for the UL141 gene. Statistical significance was determined using two-way analysis of variance, followed by Bonferroni’s posthoc test (P values are shown). Peripheral blood was harvested from mock-infected huNSG mice (n = 5), huNSG mice latently infected with WT HCMV (n = 4), or HCMVΔUL7-infected huNSG mice (n = 5) at 8 weeks postinfection and analyzed by flow cytometry for the relative percentage of human CD14+ monocytes (C), CD3+ T cells (D), and CD19+ B cells (E). All samples were treated with red blood cell (RBC) lysis buffer, blocked with human and mouse serum, and stained with antibodies specific for human CD3, CD14, CD19, and CD45, and mouse CD45. Data shown for each individual huNSG mouse were gated on total, viable, mouse CD45−, human CD45+ leukocytes. Error bars represent standard errors of the means. Statistical significance was determined using one-way analysis of variance, followed by Tukey’s posthoc test (P values are shown; NS, not significant).