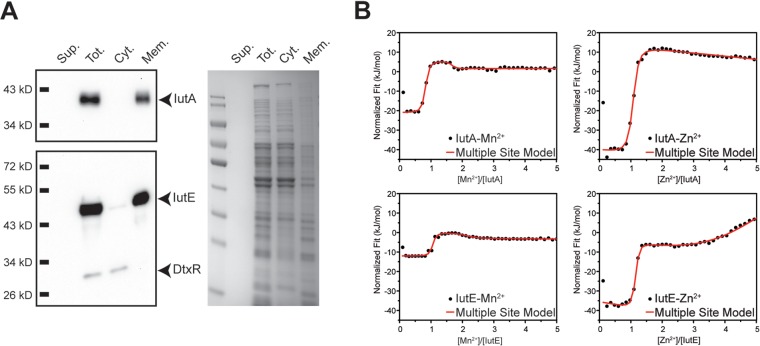
FIG 2.
Subcellular fractionation and ITC for IutA and IutE. (A) Wild-type C. diphtheriae proteins were separated following lysis and ultracentrifugation. Filtered culture supernatant (Sup.), total lysate (Tot.), and the cytoplasmic (Cyt.) and membrane (Mem.) fractions were probed using antisera against IutA, IutE, and DtxR or stained with Coomassie blue. (B) Representative fitted ITC binding profiles for IutA and IutE with Mn2+ and Zn2+. For Mn2+ binding to IutA and IutE (left upper and lower panels, respectively), two binding sites are evident for each protein titration profile as two inflection points at ∼1- and ∼2-fold excess of Mn2+ with 1:1 protein/ligand stoichiometry. However, for Zn2+ binding to IutA and IutE, only a single high-affinity site is observed for each protein at stoichiometric ligand/protein amounts (right upper and lower panels, respectively). At >2-fold ligand excess, weak metal binding is observed for both proteins as evidenced by a negative slope and a positive slope for IutA and IutE, respectively. Thermodynamic parameters obtained after fittings are shown in Table 1.