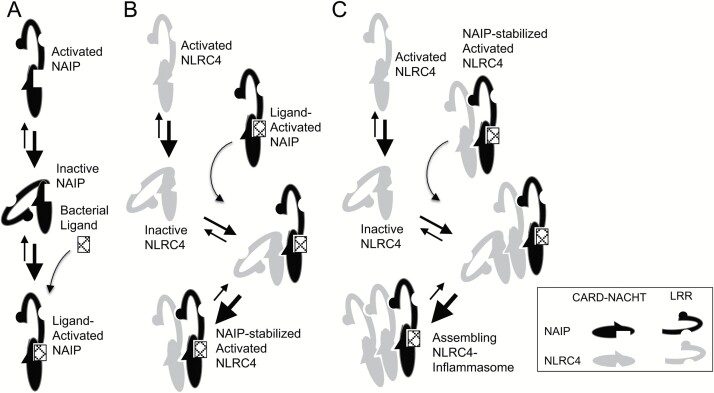
Fig. 2.
A model for assembly of the NLRC4 inflammasome includes sequential association with inactive NLR monomers followed by stabilization of the activated conformation of the added monomer. (A) NAIP favors its inactive conformation at baseline. In the presence of appropriate bacterial ligand, NAIP is stabilized in its open, activated conformation. (B) Ligand-mediated activation of NAIP uncovers an NLRC4-interacting surface on the NACHT domain, which binds the inactive NLRC4 protomer. Like NAIP, unliganded NLRC4 favors its inactive conformation but, once bound to NAIP, the open NLRC4 conformation is stabilized through intermolecular LRR interactions. (C) Activation of NLRC4 reveals a previously buried homo-oligomerization surface, which can interact with both active and inactive NLRC4 conformations. Once associated with an active NLRC4 monomer, the newly added NLRC4 is also stabilized in its active conformation, which can now associate with the next inactive NLRC4 monomer. Step C is repeated until inflammasome assembly is complete.