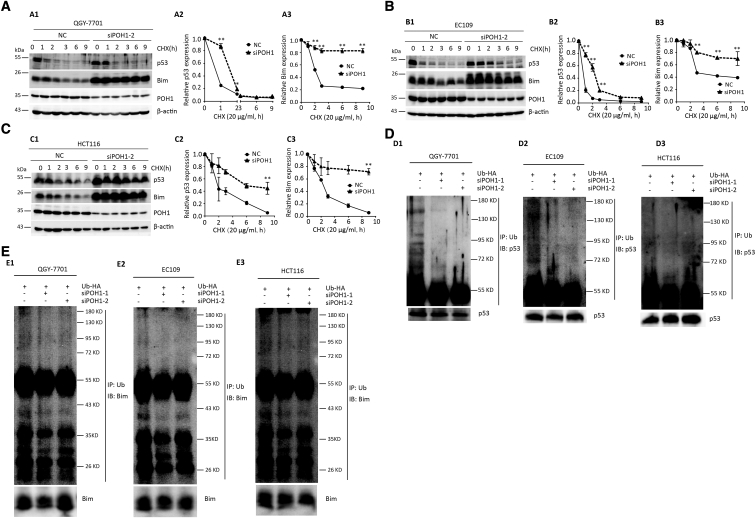
Figure 5.
POH1 knockdown attenuates the degradation of p53 and Bim. POH1 contributed to the stability of p53 and Bim proteins. (A) QGY-7701 cells with POH1 siRNAs were treated with 20 μg/ml CHX for indicated periods. The expression of POH1, p53, and Bim was detected (A1). The decrease in p53 (A2) and Bim (A3) proteins was normalized and shown. (B) EC109 cells with POH1 siRNAs were treated with 20 μg/ml CHX for indicated periods. The expression of POH1, p53, and Bim was detected (B1). The decrease in p53 (B2) and Bim (B3) proteins was normalized and shown. (C) HCT116 cells with POH1 siRNAs were treated with 20 μg/ml CHX for indicated periods. The expression of POH1, p53, and Bim was detected (C1). The decrease in p53 (C2) and Bim (C3) protein was normalized and shown. β-Actin was used as a loading control. (D) POH1 knockdown attenuated p53 ubiquitination. Cells were transfected with combinations of plasmids encoding HA-Ub, POH1 siRNA, or scramble siRNA and treated with MG132 9 hours before the ubiquitination assay. (E) POH1 knockdown attenuated Bim ubiquitination. Cells were transfected with combinations of plasmids encoding HA-Ub, POH1 siRNA, or scramble siRNA and treated with MG132 9 hours before the ubiquitination assay. Bound and input proteins were detected by immunoblotting using antibodies as indicated. At least three experiments were performed. Data are mean ± SD (All *P<.05 and **P<.01).