Abstract
Objectives
Pediatric colonic eosinophilia represents a confounding finding with a wide differential. It is often difficult to determine which children may progress to inflammatory bowel disease (IBD), which have an eosinophilic colitis (EC), and which may have no underlying pathology. There is little guidance for the practitioner on the approach to these patients. To define the clinical presentations of colonic eosinophilia and identify factors which may aid in diagnosis we reviewed patients with colonic eosinophilia and the clinicopathologic factors associated with their diagnoses.
Methods
An 8-year retrospective chart review of children whose histopathology identified colonic eosinophilia (N=72) compared to controls with normal biopsies (N=35).
Results
Patients with colonic eosinophilia had increased eosinophils/high power field (eos/HPF) compared to controls (p<0.001) and had three clinical phenotypes. Thirty six percent had an inflammatory phenotype with elevated ESR (p < .0001), chronic inflammation on colonic biopsies (p<0.001) and were diagnosed with IBD. Thirty seven percent were diagnosed with EC, associated with male gender (p <0.005) and peripheral eosinophilia (p=0.041). Twenty one percent had no significant colonic pathology. Forty three percent of patients had more than one colonoscopy and 68% of these had change from initial diagnoses.
Conclusions
There are three main phenotypes of children with colonic eosinophilia. Signs of chronic systemic inflammation raise suspicion for IBD. Peripheral eosinophilia and male gender are associated with EC. A significant percent of children with colonic eosinophilia do not have colonic disease. Eos/HPF is not reliable to differentiate etiologies. Repeat colonoscopies may be required to reach final diagnoses.
Keywords: Eosinophilic Colitis, Inflammatory Bowel Disease, Eosinophil
Introduction
Colonic eosinophilia represents a challenging histological feature observed in some pediatric patients undergoing evaluation for common gastrointestinal complaints. Pathological guidelines distinguishing normal from abnormal numbers of colonic eosinophilia are scarce and have found variable results with mean maximum eosinophils/high powered field (eos/HPF) in the cecum from 14 to 471–4 in normal children, and geographic variation has been described as well5. Little is known about the prognosis or pathogenesis of this finding in the absence of some clear causes such as parasitic infection, drug reaction, bone marrow transplant, collagen vascular disease, radiation treatment, or constipation6.
Children and adolescents with colonic eosinophilia often do not respond to dietary restriction7 suggesting that food allergy is not the primary driver of colonic eosinophilia in these patients. However, in infants colonic eosinophilia appears to have a defined allergic reaction8, 9. Clinical experiences suggest that colonic eosinophilia may be a subset of inflammatory bowel disease (IBD)10–13. Other studies describe colonic eosinophilia as preceding11 or overlapping14 the diagnosis of IBD, yet this has not been well defined15. Another group of patients with colonic eosinophilia, without evidence of IBD or other known cause, has been described and termed eosinophilic colitis (EC). Adult series suggest EC can be either a self-limited condition or have a waxing and waning course16. Significant difficulties arise in differentiating EC from IBD as they can present with similar symptoms. Previous studies have not delineated clinicopathologic factors which may help the clinician to distinguish between this diverse group of diseases. To better understand the clinical ramifications of colonic eosinophilia, we performed a retrospective analysis of children seen at a single institution who had an initial pathological report of “colonic eosinophilia”. The aims of our study were; 1) to compare control colon biopsies to patients with colonic eosinophilia to better define colonic eosinophilia, 2) to delineate clinicopathologic factors which may help identify patients with IBD vs. EC vs. other diagnoses, 3) to determine if repeat colonoscopy clarified the diagnoses.
Materials and Methods
Subject selection
A search of the Children’s Hospital Colorado Department of Pathology database from 2006–2013 was conducted using the search terms “Colonic eosinophilia”, “Eosinophilic colitis” “Eosinophilia” or “Increased Eosinophils”. Because there is no agreed upon definition for normal vs. abnormal numbers of eosinophils in the pediatric colon, we did not use a specific number cutoff of eosinophils to select subjects but instead reviewed all records in which pathologist’s interpretation was stated to be one of these above terms. We identified this cohort as having “colonic eosinophilia”.
A similar search of the Children’s Hospital Colorado Department of Pathology database was conducted to identify controls. These control subjects were previously identified at our institution17, had common symptoms of gastrointestinal dysfunction (abdominal pain, diarrhea, concern for juvenile polyp). They underwent upper endoscopy and colonoscopy without endoscopic or histologic pathology (except juvenile polyp), without mention of increased eosinophils or any features of acute or chronic inflammation. There was no evidence of elevated inflammatory markers (ESR, CRP), anemia or history of any use of medications likely to alter eosinophil numbers in the colon (systemic steroids, 5-aminosalicylates, immunomodulators, biologic therapies, or antibiotics). We termed this cohort “controls”.
Electronic medical records review (EMRs) identified clinical features of control subjects and those with colonic eosinophilia, which included demographics, symptoms, laboratory testing, endoscopic findings and final diagnoses.
Ulcerative colitis (UC), Crohn’s disease (CD), and indeterminate colitis were combined for a diagnosis of “Any IBD” and separated into discrete categories. Laboratory studies performed within 1 month of colonoscopy were included in analysis.
Exclusion criteria included: 1. Under 1 year of age at initial biopsy-diagnoses of allergic colitis, 2. Bone marrow transplant or 3. Parasitic infection. This study was approved by the Institutional Review Board at the University of Colorado.
Specimen Histological Assessments
All hematoxylin and eosin (H&E) stained colonic tissue sections from “colonic eosinophilia” subjects were assessed by a board certified pediatric pathologist (KC). Signs of chronic inflammation on histopathology were identified by the pathologist (KC) when distorted gland architecture, multiple branched glands, Paneth cells in the left colon and/or fibrosis were present. Entire specimens were reviewed and the area with highest eosinophil density was selected to enumerate peak number of eosinophils/high powered field (eos/HPF) at ×40 magnification field size of 0.26 mm2.
Colonic biopsies from control subjects were evaluated in a similar fashion by the pathologist (KC) as well as 4 independent observers (ED, JM, JM, SF). Using 92 pathological slides from 35 controls, inter-observer reliability between these observers and KC was evaluated by pair-wise Bland-Altman plot analysis. Intra-class correlation coefficient was greater than 0.85 suggesting strong agreement.
Statistical analysis
SAS 9.4 (SAS Institute Inc., Cary, NC, USAS) was used for all the analyses. Associations of max eos/HPF with other continuous variables were assessed using spearman correlation. Two independent sample t-test and Chi square test were used respectively to compare continuous and categorical outcomes between controls and patients with eosinophilia and between patients with and those without IBD diagnosis. A linear mixed effects model with unstructured covariance was used to compare max eos across three segments of colon and between patients with and without IBD. P value less than 0.05 was deemed to be significant.
Results
Patient demographics and history
Review of our pathology database identified 78 patients with colonic eosinophilia. Six were not included based on exclusion criteria. Comparison of colonic eosinophilia patients to controls revealed that they were younger at the first colonoscopy, more often reported a history of environmental allergies, eczema, asthma and a family history of allergic and/or gastrointestinal disease (Table 1).
Table 1.
General Patient Demographics and History comparing control biopsies to those with colonic eosinophilia
| Controls (N=35) | Colonic Eosinophilia (n=72) | p-value | |
|---|---|---|---|
| Female/Male (% Male) | 20/15 (42.8) | 30/42 (58) | 0.13 |
| Average age at 1st colonoscopy in years | 11.37 (5.09) | 8.92 (5.37) | 0.0224 |
| Average number colonoscopies | 1 | 1.88 | |
| % with environmental/food or drug allergies | 25.71 | 61.11 | <0.001 |
| % with asthma | 2.86 | 34.72 | <0.001 |
| % with eczema | 11.4 | 25.00 | 0.07 |
| % with family history of GI illness | 14.29 | 37.50 | 0.008 |
Evaluation of eos per HPF in the colon
We next assessed the peak mucosal eosinophils in those with colonic eosinophilia and normal controls at each section of the colon and compared the means of peak counts of different groups (Figure 1). Patients with colonic eosinophilia had significantly greater mean numbers of eos/HPF in the cecum/ascending colon (colonic eosinophilia 56.0 +/− 20.5, control 24.2 +/− 10.2,) transverse/descending (colonic eosinophilia 46.7 +/− 23.1, control 18.8 +/− 8.1) and recto sigmoid colon (colonic eosinophilia 43.2 +/− 24.6, control 11.7 +/− 7.1) p< 0.001 for differences in means for all locations.
Figure 1.
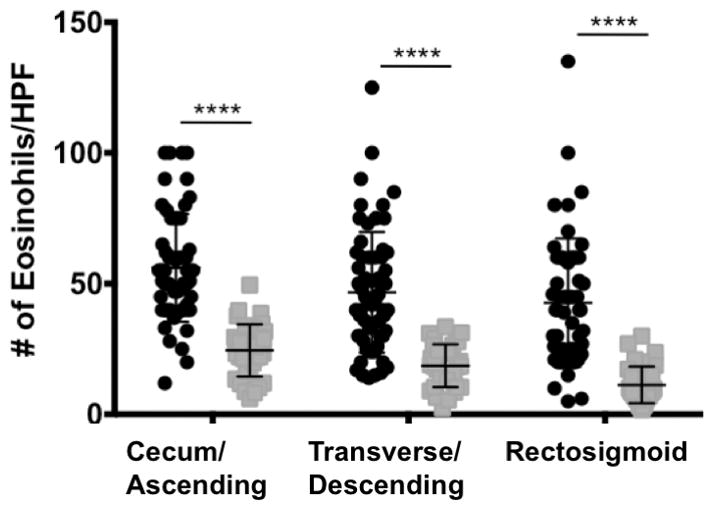
Black error bar identifies Mean peak eosinophils/HPF (eos/HPF) in control colonic (gray square) biopsies vs. patients with colonic eosinophilia (black circle). **** p<.0001
Diagnosis and presentation of Colonic Eosinophilia
Patients with colonic eosinophilia presented with a variety of symptoms. The three most common of which included abdominal pain (59%), hematochezia (47%) and diarrhea (39%) (Figure 2A). Patients with known or suspected eosinophilic esophagitis (EoE) underwent colonoscopy for symptoms raising suspicion for colonic dysfunction (lower abdominal pain, hematochezia, diarrhea). A final diagnosis of eosinophilic gastrointestinal disease (EGID or EC) (EC, with or without enteritis, or gastritis) was seen in 27/72 patients. IBD (CD (N=10), UC (N=6) or IC (N=10)) was found in 26/72 (36%) patients. A combination of other diagnoses (other) such as toddler’s diarrhea, constipation, collagenous colitis, lymphocytic colitis, irritable bowel syndrome (IBS), no colonic pathology, eosinophilic esophagitis (EoE) and unknown/lost to follow up were identified in 35/72 (49%). (Figure 2B). Median follow up was 12 months, range 0–84 months. Some patients had multiple diagnoses which were not mutually exclusive. We next focused our assessment to understand differences between patients presenting with colonic eosinophilia who ultimately received diagnoses of IBD, EC, or other diagnoses.
Figure 2. A) Presenting symptoms of patients with colonic eosinophilia (percent). B) Final diagnoses of patients noted to have colonic eosinophilia (percent). Other includes.
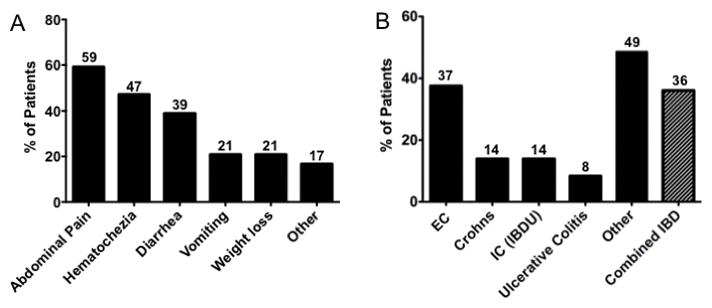
irritable bowel syndrome, EoE, lymphocytic colitis, constipation, toddler’s diarrhea (some patients received more than one final diagnosis such as EoE and EC).
Evaluation of Colonic Eosinophilia in IBD vs. other diagnoses
As a primary differentiating step, we compared colonic eosinophilia between patients with IBD and patients without IBD. We found that mean maximum eos/HPF were not significantly different between the two groups in the cecum/ascending colon (62.5 +/− 20.3 vs. 52.4 +/− 20.0 p= 0.078) or transverse/descending colon (53.9 +/− 27.0 vs. 42.6 +/− 19.75, p= 0.065), but were in the rectosigmoid colon (56.6 +/− 28.1 vs. 37.5 +/− 20.9 p= 0.0081). However, there was significant overlap between the two groups in all locations (Figure 3).
Figure 3.
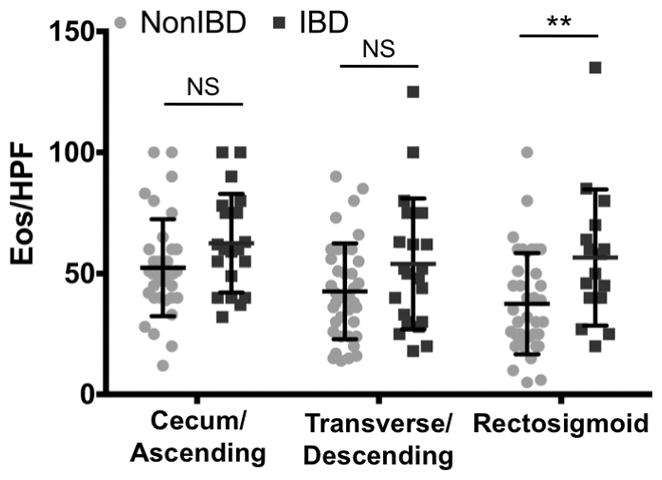
Peak eos/HPF by location in the colon in patients with colonic eosinophilia with diagnosis of inflammatory bowel disease (IBD) and without IBD (Non-IBD). Differences in peak mean eos/HPF were significantly different in the rectosigmoid (p = 0.008)
We next evaluated clinicopathologic factors in patients with final diagnoses of IBD vs. other diagnoses (Table 2). Significant differences between groups included: age at biopsy (p= 0.0003), hemoglobin level (p= 0.0217), with corresponding difference in hematocrit, erythrocyte sedimentation rate (p < 0.0001), percent of patients who presented with: hematochezia (p= 0.0002), vomiting (p= 0.0065), and signs of chronic colitis on initial biopsy (p < 0.001). Signs of chronic colitis on biopsy was the most sensitive marker for IBD with only 4 patients who did not have chronic changes on initial colonic biopsy who were diagnosed with IBD. 3 of these patients were diagnosed with Crohn’s disease because of findings in the small bowel consistent with Crohn’s disease. We found that 41% of patients not diagnosed with IBD had a concurrent diagnosis of EoE whereas no patients with IBD met criteria for EoE (p= 0.0001). No significant differences were found in gender, white blood cell count (WBC), peripheral blood eosinophil count, C-reactive protein (CRP), serum albumin, or presenting symptoms of diarrhea, abdominal pain, weight loss/poor weight gain, or location of eosinophils (confined to the lamina propria compared to infiltrating the epithelium and/or crypts). Finally (Table S1), we evaluated the relationship of number of eos/HPF to the same variables as in Table 2 and determined that patients with hematochezia and vomiting had significantly different numbers of eosinophils in the colon.
Table 2.
Selected clinicopathologic factors in patients with IBD vs. patients without IBD. Patients with IBD were significantly older, had higher ESR, lower hemoglobin, more likely to have chronic inflammation on colonic biopsy, were less likely to present with vomiting and less likely to have diagnosis of EoE. Number of patients (N), Standard deviation (SD)
| IBD N=26 |
Non IBD N=46 |
P value | |
|---|---|---|---|
| Age at Biopsy (yrs) [Mean (SD)] | 11.88 (4.89) | 7.24 (4.93) | 0.0003 |
| % male | 53.8 | 60.9 | 0.56 |
| Number of patients presenting with hematochezia [N (%)] | 20 (76.9) | 14 (31.1) | 0.0002 |
| Patients presenting with vomiting [N (%)] | 1 (3.8) | 14 (31.1) | 0.0065 |
| Patients presenting with diarrhea [N (%)] | 7 (26.9) | 21 (46.7) | 0.1 |
| Patients presenting with abdominal pain [N (%)] | 16 (61.5) | 26 (57.8) | 0.76 |
| Peak eosinophilia/HPF [Mean (SD)] | 66.77 (24.60) | 56.17 (22.17) | 0.065 |
| Eosinophils in the epithelium [N (%)] | 14 (53.8) | 19 (41.3) | 0.304 |
| Patients presenting with weight loss/poor weight gain [N (%)] | 5 (19.2) | 10 (22.2) | 0.77 |
| Patients with signs of chronic colitis on initial biopsy [N (%)] | 22 (84.6) | 10 (21.7) | <0.001 |
| Patients with the diagnosis of Eosinophilic esophagitis [N (%)] | 0 (0) | 19 (41.3) | <0.0001 |
| WBC [Mean (SD)] | 9.00 (3.10) | 8.54 (3.66) | 0.64 |
| Hgb [Mean (SD)] | 12.33 (2.28) | 13.65 (1.55) | 0.022 |
| Peripheral blood eosinophil count [Mean (SD)] | 415.10 (434.56) | 659.68 (848.24) | 0.18 |
| ESR [Mean (SD)] | 22.43 (12.98) | 6.75 (5.18) | <.0001 |
| CRP [Mean (SD)] | 0.66 (0.75) | 0.37 (0.55) | 0.18 |
| Albumin [Mean (SD)] | 3.88 (0.55) | 3.97 (0.58) | 0.58 |
Comparison of patients without IBD, EC/EGID vs. all other diagnoses
As a secondary differentiating step, patients with colonic eosinophilia without IBD were further evaluated to attempt to define their diagnoses (Table 3). Significant differences between groups (N=27 for EGID/EC and N=19 for non-IBD, non-EGID) included male gender (77.8 vs. 36.8% p = 0.005), and mean peripheral blood eosinophil count (820 vs. 323 cells/μL p = 0.041). No other significant differences were found in clinical, lab, or histologic factors assessed. Of the 19 patients with colonic eosinophilia without diagnosis of IBD or EC, 15 of 19 eventually were determined to not have colonic pathology. Seven were ultimately diagnosed with EoE based on symptoms of esophageal dysfunction and histologic findings of > 15 eosinophils per HPF in esophageal biopsies after proton pump inhibitor trials, and no colonic pathology based on resolution of eosinophilia on repeat colonoscopy and/or resolution of any lower GI symptoms at the end of follow up period. Five were diagnosed with functional GI disorders, 2 with constipation, 1 with toddler diarrhea which resolved with time. Two of 19 were diagnosed with rarer types of colitis (1 lymphocytic and 1 collagenous colitis), and 2 were lost to follow up (Table 4). This analysis showed that 15 (21%) of the initial 72 patients with colonic eosinophilia did not have significant colonic pathology after clinical and endoscopic follow up.
Table 3.
Selected clinicopathologic factors in patients with colonic eosinophilia diagnosed with eosinophilic colitis/eosinophilic gastrointestinal disease (EC/EGID) vs. patients not diagnosed with either IBD or EC/EGID (non-IBD, non-EC/EGID). Patients with EC/EGID were significantly more likely to be male and have elevated eosinophils in peripheral blood. Number of patients (N), standard deviation (SD).
| Eosinophilic Colitis N=27 |
Non-Eosinophilic colitis/Non-IBD N=19 |
P value | |
|---|---|---|---|
| Age at biopsy (yrs) [mean (SD)] | 6.67 (4.83) | 8.05 (5.08) | 0.35 |
| % male | 77.78 | 35.8 | 0.005 |
| Number of patients with signs of chronic colitis on initial biopsy [N (%)] | 10 (29.63) | 2 (10.53) | 0.12 |
| Number of patients presenting with hematochezia [N (%)] | 9 (33.33) | 5 (26.32) | 0.61 |
| Number of patients presenting with vomiting [N (%)] | 9 (33.33 | 5 (26.32) | 0.61 |
| Number of patients presenting with diarrhea [N (%)] | 13 (48.15) | 8 (42.11) | 0.69 |
| Number of patients with abdominal pain [N (%)] | 13 (48.15) | 13 (68.42) | 0.17 |
| Number of patients with weight loss/poor weight gain [N (%)] | 6 (22.22) | 4 (21.05 | 0.93 |
| Number of patients with the diagnosis of Eosinophilic esophagitis [N (%)] | 12 (44.4) | 7 (36.8) | 0.6 |
| WBC [Mean (SD)] | 8.77 (3.99) | 8.01 (2.96) | 0.54 |
| Hgb [Mean (SD)] | 13.63 (1.73) | 13.67 (1.18) | 0.95 |
| Peripheral blood eosinophil count [Mean SD)] | 820 (979) | 323 (279) | 0.042 |
| ESR [Mean (SD)] | 6.45 (5.62) | 7.5 (3.98) | 0.86 |
| CRP [Mean (SD)] | 0.35 (0.44) | 0.42 (0.83) | 0.85 |
| Albumin [Mean (SD)] | 3.91 (0.69) | 4.11 (0.27) | 0.28 |
| Peak eosinophilia/HPF [Mean (SD)] | 54.74 (19.47) | 58.21 (25.95) | 0.63 |
| Eosinophils in the epithelium [N (%)] | 12 (44.4) | 7 (36.8) | 0.644 |
Table 4.
Diagnoses of patients with colonic eosinophilia without IBD or diagnosis of primary EC (n = 19). 7 patients had resolution of lower abdominal complaints and/or resolution of colonic eosinophilia on repeat endoscopy but met criteria for EoE (EoE). 5 patients had clinical course consistent with functional abdominal pain/IBS. 2 patients had colonic eosinophilia but known histories of constipation and symptoms resolved with treatment of constipation. 1 patient had diarrhea as a toddler which resolved with time and no intervention. 2 patients had more rare colitites (collagenous and lymphocytic colitis). 2 patients were lost to follow up (family moved out of state) < 4 weeks after endoscopy without leading diagnosis.
| Number of patients | Final diagnosis |
|---|---|
| 7 | No colonic pathology, EoE |
| 5 | Functional/IBS |
| 2 | Constipation |
| 1 | Toddler diarrhea |
| 1 | Lymphocytic colitis |
| 1 | Collagenous colitis |
| 2 | Lost to follow up |
Repeat colonoscopy for colonic eosinophilia
We determined that 31/72 (43.1%)of the patients with colonic eosinophilia found on the initial colonoscopy had diagnoses that remained uncertain and/or continued to have clinical symptoms, and underwent a second colonoscopy (Figure 4A). Sixty eight percent of patients who received 2 or more colonoscopies had a change in diagnosis (Figure 4B). Patients underwent the second colonoscopy on average 1.14 +/− 0.96 years after the first colonoscopy. Analysis of this subgroup revealed that, after the first colonoscopy, 11 patients had an undefined diagnosis, 10 patients had diagnosis of IBD and 7 had an original diagnosis of EGID/EC. After repeat colonoscopies, these numbers changed to 0 undefined, 14 with IBD and 10 with EGID/EC, and 4 patients without signs or symptoms of colonic pathology (Figure 4C).
Figure 4.
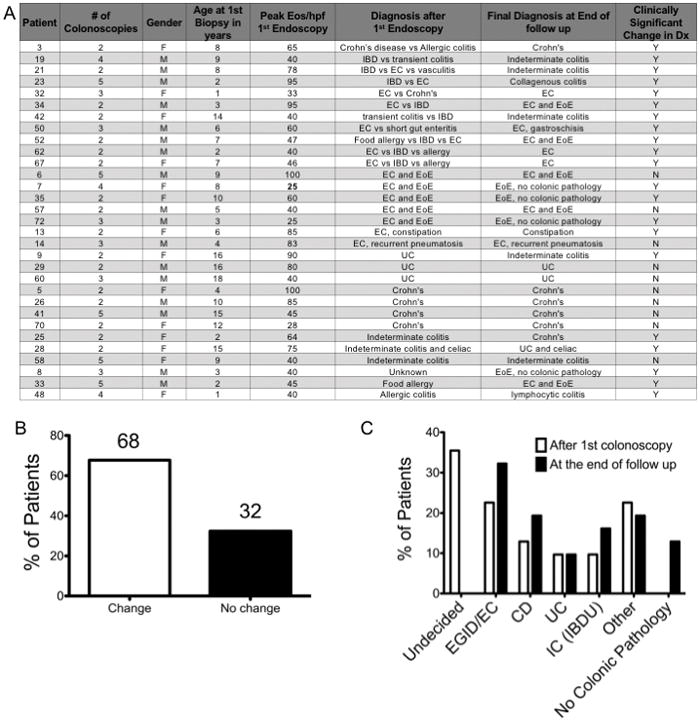
A) Patient data for patients undergoing at least 2 colonoscopies B) Percent of patients with a clinically relevant change in diagnosis after repeat endoscopies. C) Diagnoses of patients after the first endoscopy and after 2 or greater endoscopies. Patients who had more than one possible diagnosis considered were labeled as Undecided. Several patients had multiple diagnoses that were not mutually exclusive and included in other.
Discussion
The aim of our study was to aid clinicians in understanding pediatric colonic eosinophilia. A major question in pediatrics is: how to define pathologic colonic eosinophilia, and can the number of eos/HPF delineate etiology? We found a significant difference in the mean of the peak eos/HPF in all locations in the colon between patients with colonic eosinophilia and controls. However, one novel aspect of our study is the significant variability found in controls and in children with colonic eosinophilia and the overlap of peak eos/HPF between control patients and those thought to have colonic eosinophilia. This variability likely contributes to the lack of consensus of normal versus abnormal eos/HPF. Different published studies have found different numbers of eos/HPF in healthy children as well as a decrease in eos/HPF as one moves distally along the colon1–3, 18, 19, which we have confirmed. As there is no consensus for pathologic colonic eosinophilia versus normal variation, we did not rely on a number cutoff to identify patients but rather pathologist interpretation of the biopsies as this is the situation frequently encountered by clinicians based on current knowledge. We were unable to define a clinically useful number cutoff for normal and abnormal eos/HPF given the significant overlap between our normal controls and patients with colonic eosinophilia. Any number chosen based on our sample would either misidentify many normal patients as abnormal or fail to identify many patients with pathologic colonic eosinophilia.
At first glance our study appears to have different results for the control biopsies compared to a recent cohort of healthy Canadian children4. Our numbers of mean peak eos/HPF of 24.2, 18.8, and 11.7 for the cecum/ascending, transverse/descending, and rectosigmoid colon respectively are approximately half those found in the Canadian study, however the HPF area for their study was 0.55 mm2 and our HPF area was roughly half at 0.26 mm2. Our study has similar results to DeBrosse et al 20062 in the control subjects with their peak means 20.3, 16.3, and 8.3 for the ascending, transverse colon, and rectum respectively. Their HPF area was 0.28 mm2. The slight difference in means is likely accounted for by the variation in healthy patients and slightly different biopsy locations between studies. This highlights two difficulties in interpreting eos/HPF between published studies. The biopsy sites may be different, and there is no standardization for the area of HPFs. This places the responsibility on the clinician to confirm HPF area at their institution before interpreting eos/HPF results in relation to published studies, unless HPF areas can be standardized in the future. Overall our analysis of control patients compared with those with colonic eosinophilia leads to the conclusion that eosinophils should be enumerated on biopsy reports to help guide the practitioner, but that the number of eos/HPF is not sufficient for diagnosis and other clinical factors must be considered.
We found 3 main groups of patients with colonic eosinophilia, which are important to distinguish: IBD, EC, and no significant colonic pathology. For the clinician, a major question that will guide future management for a patient with colonic eosinophilia is if the child has IBD. IBD was diagnosed in 36% of patients in our sample, which is similar to what has been described previously19. The IBD group is characterized by an older age at presentation (mean 11.88 years versus 7.25) a significant inflammatory picture with elevated ESR, chronic inflammatory changes on colonic biopsy and hematochezia. No single factor reliably predicted diagnosis of IBD, but the whole patient presentation was considered. Even chronic changes on initial biopsy was not specific as 21.7% of patients without IBD had chronic changes on initial biopsy. Of these, 8 patients were diagnosed with EC as their biopsies were not classic for IBD given the eosinophilic predominance of their inflammation, 1 was diagnosed with collagenous colitis, and 1 had resolution of both chronic changes and colonic eosinophilia on repeat colonoscopies. We found that eos/HPF, CRP, albumin, and weight loss were not reliable ways to identify patients with IBD. Unfortunately, too few of the patients we evaluated had fecal calprotectin testing performed making it impossible to analyze this as a diagnostic test to assist in the differentiation of IBD and other causes of colonic eosinophilia. Interestingly, patients with IBD were less likely to present with vomiting. This is likely related to 41% of patients in the non-IBD group also were diagnosed with EoE compared to none of the patients with IBD. This could help the clinician, in that, if a patient has colonic eosinophilia and meets criteria for EoE, it is unlikely that IBD is the underlying etiology, and primary EC or another etiology should be considered.
If a patient with colonic eosinophilia does not fit the clinical picture for IBD, the next important populations to distinguish are EC from other etiologies. We found that the factors associated with EC included higher peripheral eosinophil counts and male gender. These associations have previously been described in patients with eosinophilic gastrointestinal diseases20–22 and warrant additional studies. Twenty one percent of patients identified with colonic eosinophilia did not have significant colonic pathology based on resolution of lower GI symptoms or normal histology on repeat colonoscopies. Again, this highlights that the number of eos/HPF alone is not a reliable indicator for underlying etiology and the whole clinicopathologic picture must be considered. Since a significant proportion of patients with colonic eosinophilia may not have significant underlying colonic disease, the diagnostic and treatment approach must be tailored to the individual patient. Another consideration is that IBD may have years of quiescence between flares. It is possible that some patients who were identified as colonic eosinophilia with subsequent symptomatic resolution may go on to develop more classic signs and histology of IBD in the future, but this would need to be evaluated in further long term studies.
There were many patients for whom the diagnosis after initial colonoscopy was unclear or who had continued symptoms. This prompted 43% to undergo at least one additional colonoscopy. In these patients, the diagnosis significantly changed (unclear to more clear diagnosis, change of initial diagnoses to alternate diagnoses) in 68% of patients, and the percent of patients with 2 or more diagnoses being considered decreased to 0. In children, the decision to repeat an invasive procedure is not taken lightly given concern over neurodevelopmental effects of repeat or prolonged anesthesia episodes23–25, potential for adverse events, and significant cost. However, we conclude from our study that in patients with colonic eosinophilia in whom the diagnosis is unclear or who are not improving, repeat colonoscopy is a reasonable step as in 2/3 of patients it can lead to a significant change in diagnosis and subsequent treatment.
Our study has several strengths: it is the largest population of pediatric patients with colonic eosinophilia to be studied, the only one to compare controls to colonic eosinophilia, evaluate clinicopathologic findings associated with colonic eosinophilia and examine the utility of repeat colonoscopy. Limitations to this study include that it was performed in a single referral center for eosinophilic gastrointestinal diseases and the study design was retrospective. In addition, we were unable to reliably determine use of medications at the time of colonoscopy. This study raises questions for future research including how to define pathologic colonic eosinophilia more reliably than eos/HPF. The lack of consensus on definition of pathologic colonic eosinophilia does not allow identification of a number cutoff of eos/HPF but rather the pathologist interpretation of increased eosinophils. We see from our population that choosing a number cutoff alone would not reliably differentiate normal from pathologic. Future research should move away from trying to define a normal number of eos/HPF and shift to focus on what other histologic factors may be a more reliable way to differentiate. Eotaxin staining, presence of eosinophil degranulation and IL-5 staining are some possibilities. Until more reliable factors are identified, there will continue to be significant difficulty in defining pathologic colonic eosinophilia.
Supplementary Material
a) Association between maximum eos/HPF on colonic biopsy with continuous clinical variables. Negative spearman r denotes a higher max eos/HPF correlates with a decrease in the other variable. b) Association of maximum eos/HPF and categorical variables. Mean eos/HPF between groups compared with 2 sample t-tests. Significant associations included higher eos/HPF were associated with hematochezia, diagnosis of indeterminate colitis, and signs of chronic colitis on initial biopsy. (SD=Standard deviation).
What is known
Colonic eosinophilia in pediatrics is not well defined
Peak number of Eosinophils is variable thus not diagnostic
IBD has been associated with eosinophilia
What is new
3 phenotypes of Colonic Eosinophilia are identified
Association of Age, sex and chronic disease with Colonic Eosinophilia
Need for repeat colonoscopy to define diagnosis
Acknowledgments
Support: NIH 1K24DK100303 (Furuta GT) and Consortium for Gastrointestinal Eosinophilic Researchers (CEGIR). CEGIR (U54 AI117804) is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. (Furuta GT)
Footnotes
Disclosures: No conflicts of interest exist
Author Contributions:
Jacob Mark M.D. This author was involved in conception and design of the work; acquisition, analysis, and interpretation of data for the work; editing and proofing of this manuscript
Shahan D. Fernando M.D. This author was involved in conception and design of the work; acquisition, analysis, and interpretation of data for the work; editing and proofing of this manuscript
Joanne C. Masterson Ph.D. This author was involved in design of the work; acquisition, analysis, and interpretation of data for the work; editing and proofing of this manuscript
Zhaoxing Pan M.B., Ph.D. This author was involved in analysis, and interpretation of data for the work; editing and proofing of this manuscript.
Kelley E. Capocelli M.D. This author was involved in acquisition, analysis, and interpretation of data for the work editing and proofing of this manuscript
Glenn T. Furuta M.D. This author was involved in conception and design of the work; acquisition, analysis, and interpretation of data for the work; editing and proofing of this manuscript
Edwin F. de Zoeten M.D., Ph.D. This author was involved in conception and design of the work; acquisition, analysis, and interpretation of data for the work; editing and proofing of this manuscript
References
- 1.Saad AG. Normal quantity and distribution of mast cells and eosinophils in the pediatric colon. Pediatr Dev Pathol. 2011;14:294–300. doi: 10.2350/10-07-0878-OA.1. [DOI] [PubMed] [Google Scholar]
- 2.DeBrosse CW, Case JW, Putnam PE, et al. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9:210–8. doi: 10.2350/11-05-0130.1. [DOI] [PubMed] [Google Scholar]
- 3.Lowichik A, Weinberg AG. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol. 1996;9:110–4. [PubMed] [Google Scholar]
- 4.Chernetsova E, Sullivan K, de Nanassy J, et al. Histologic analysis of eosinophils and mast cells of the gastrointestinal tract in healthy Canadian children. Hum Pathol. 2016;54:55–63. doi: 10.1016/j.humpath.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Pascal RR, Gramlich TL, Parker KM, et al. Geographic variations in eosinophil concentration in normal colonic mucosa. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1997;10:363–365. [PubMed] [Google Scholar]
- 6.Alfadda AA, Storr MA, Shaffer EA. Eosinophilic colitis: epidemiology, clinical features, and current management. Therap Adv Gastroenterol. 2011;4:301–9. doi: 10.1177/1756283X10392443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz AJ, Twarog FJ, Zeiger RS, et al. Milk-sensitive and eosinophilic gastroenteropathy: similar clinical features with contrasting mechanisms and clinical course. J Allergy Clin Immunol. 1984;74:72–8. doi: 10.1016/0091-6749(84)90090-3. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff SC. Food allergy and eosinophilic gastroenteritis and colitis. Curr Opin Allergy Clin Immunol. 2010;10:238–45. doi: 10.1097/ACI.0b013e32833982c3. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff SC, Ulmer FA. Eosinophils and allergic diseases of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2008;22:455–79. doi: 10.1016/j.bpg.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Katsanos KH, Zinovieva E, Lambri E, et al. Eosinophilic-Crohn overlap colitis and review of the literature. J Crohns Colitis. 2011;5:256–61. doi: 10.1016/j.crohns.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Uzunismail H, Hatemi I, Dogusoy G, et al. Dense eosinophilic infiltration of the mucosa preceding ulcerative colitis and mimicking eosinophilic colitis: report of two cases. Turk J Gastroenterol. 2006;17:53–7. [PubMed] [Google Scholar]
- 12.Woodruff SA, Masterson JC, Fillon S, et al. Role of eosinophils in inflammatory bowel and gastrointestinal diseases. J Pediatr Gastroenterol Nutr. 2011;52:650–61. doi: 10.1097/MPG.0b013e3182128512. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff SC, Wedemeyer J, Herrmann A, et al. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 1996;28:1–13. doi: 10.1046/j.1365-2559.1996.262309.x. [DOI] [PubMed] [Google Scholar]
- 14.Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID) J Allergy Clin Immunol. 2004;113:11–28. doi: 10.1016/j.jaci.2003.10.047. quiz 29. [DOI] [PubMed] [Google Scholar]
- 15.Choy MY, Walker-Smith JA, Williams CB, et al. Activated eosinophils in chronic inflammatory bowel disease. Lancet. 1990;336:126–7. doi: 10.1016/0140-6736(90)91651-p. [DOI] [PubMed] [Google Scholar]
- 16.Alfadda AA, Shaffer EA, Urbanski SJ, et al. Eosinophilic colitis is a sporadic self-limited disease of middle-aged people: a population-based study. Colorectal Dis. 2014;16:123–9. doi: 10.1111/codi.12464. [DOI] [PubMed] [Google Scholar]
- 17.Masterson JC, Capocelli KE, Hosford L, et al. Eosinophils and IL-33 Perpetuate Chronic Inflammation and Fibrosis in a Pediatric Population with Stricturing Crohn’s Ileitis. Inflammatory Bowel Diseases. 2015;21:2429–2440. doi: 10.1097/MIB.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonsalves N. Food allergies and eosinophilic gastrointestinal illness. Gastroenterol Clin North Am. 2007;36:75–91. vi. doi: 10.1016/j.gtc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Pensabene L, Brundler M-A, Bank JM, et al. Evaluation of Mucosal Eosinophils in the Pediatric Colon. Digestive Diseases and Sciences. 2005;50:221–229. doi: 10.1007/s10620-005-1586-0. [DOI] [PubMed] [Google Scholar]
- 20.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e6. doi: 10.1016/j.jaci.2011.02.040. quiz 21–2. [DOI] [PubMed] [Google Scholar]
- 21.Orenstein SR, Shalaby TM, Di Lorenzo C, et al. The spectrum of pediatric eosinophilic esophagitis beyond infancy: a clinical series of 30 children. Am J Gastroenterol. 2000;95:1422–30. doi: 10.1111/j.1572-0241.2000.02073.x. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson LB. Diffuse eosinophilic gastroenteritis: an adult form of allergic gastroenteropathy. Report of a case with probable protein-losing enteropathy. Am J Gastroenterol. 1970;54:580–8. [PubMed] [Google Scholar]
- 23.Davidson AJ, Disma N, de Graaff JC, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. The Lancet. 387:239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L. Early childhood general anaesthesia exposure and neurocognitive development. BJA: British Journal of Anaesthesia. 2010;105:i61–i68. doi: 10.1093/bja/aeq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. The Journal of the American Society of Anesthesiologists. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a) Association between maximum eos/HPF on colonic biopsy with continuous clinical variables. Negative spearman r denotes a higher max eos/HPF correlates with a decrease in the other variable. b) Association of maximum eos/HPF and categorical variables. Mean eos/HPF between groups compared with 2 sample t-tests. Significant associations included higher eos/HPF were associated with hematochezia, diagnosis of indeterminate colitis, and signs of chronic colitis on initial biopsy. (SD=Standard deviation).


