Figure 7.
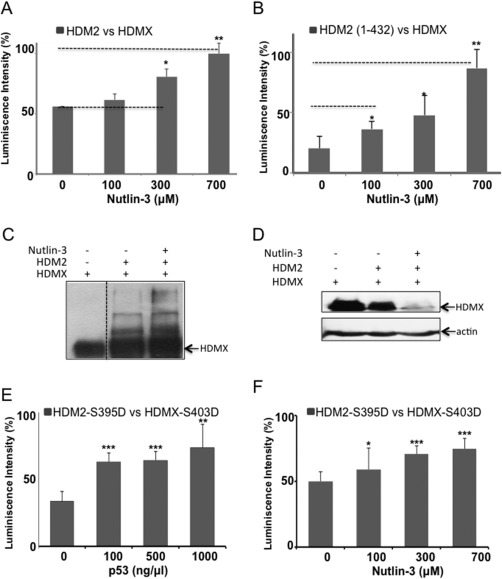
Effect of Nutlin‐3 on the HDMX‐HDM2 interaction. (A) ELISA assay using a fixed amount of HDMX (10 ng/μL) and a fixed amount of HDM2 (10 ng/μL) in the presence of different concentrations of Nutlin‐3 (0–700 μM). As with p53 protein, the presence of Nutlin‐3 increased the interaction between HDMX and HDM2. (B) ELISA assay as in (A) but using HDM2 (1–432) construct that lacks the C‐terminal domain (C) In vitro ubiquitination of recombinant HDMX with HDM2 in presence Nutlin‐3. The compound increased HMDX ubiquitination mediated by HDM2. (D) In cells ubiquitination of HDMX with HDM2 in presence of Nutlin‐3. The compound degraded HMDX even with MG132 proteasome inhibitor. The assays of ubiquitination were developed with 4.1 anti‐HDMX (E) ELISA assay using a fixed amount of HDMX(S403D) (10 ng/μL) and a fixed amount of HDM2(S395D) (10 ng/μL) in the presence of different concentrations of p53 (0–20 ng/μL). p53 increase the binding between the two phosphomimetic proteins. (F) ELISA assay using a fixed amount of HDMX(S403D) (10 ng/μL) and a fixed amount of HDM2(S395D) (10 ng/μL) in the presence of different concentrations of Nutlin‐3 (0–700 μM). As with p53 protein, the Nutlin‐3 increased the interaction between HDMX and HDM2. The assays were developed with YB25 anti‐HDM2 (*P < 0.05, **P < 0.01 and ***p < 0.001).
