Table 1. Palladium-catalyzed cross-couplings of α-trifluoromethyl-β-silyl alcohols.
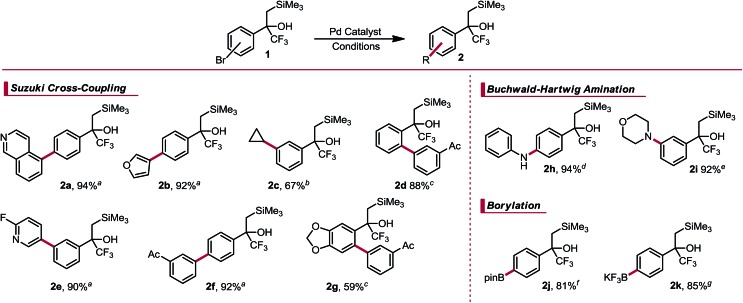
|
aReaction conditions: Pd(OAc)2 (1 mol%), RuPhos (3 mol%), organotrifluoroborate (1.1 equiv.), Na2CO3 (2 equiv.), EtOH (0.18 M), 85 °C, 24 h.
bReaction conditions: XPhos Pd G2 (3 mol%), organotrifluoroborate (1.2 equiv.), K2CO3 (3 equiv.), 10 : 1 CPME/H2O (0.25 M), 100 °C, 24 h.
cReaction conditions: Pd(OAc)2 (5 mol%), QPhos (12 mol%), organotrifluoroborate (1.1 equiv.), K2CO3 (2 equiv.), 2 : 1 dioxane/H2O (0.25 M), 85 °C, 24 h.
dReaction conditions: XPhos Pd G2 (2 mol%), aniline (3 equiv.), Cs2CO3 (1.4 equiv.), PhMe (0.5 M), 100 °C, 24 h.
eReaction conditions: XPhos Pd G2 (2 mol%), morpholine (3 equiv.), Cs2CO3 (2.5 equiv.), 5 : 1 PhMe/tBuOH (0.42 M), 80 °C, 12.5 h.
fReaction conditions: XPhos Pd G2 (2 mol%), B2pin2 (3 equiv.), KOAc (3 equiv.), dioxane (0.5 M), 110 °C, 2 h.
gReaction conditions: XPhos Pd G2 (0.5 mol%), XPhos (1 mol%), BBA (3 equiv.), KOAc (3 equiv.), EtOH (0.1 M), 80 °C then KHF2 (6.75 equiv.), MeOH (0.1 M).
