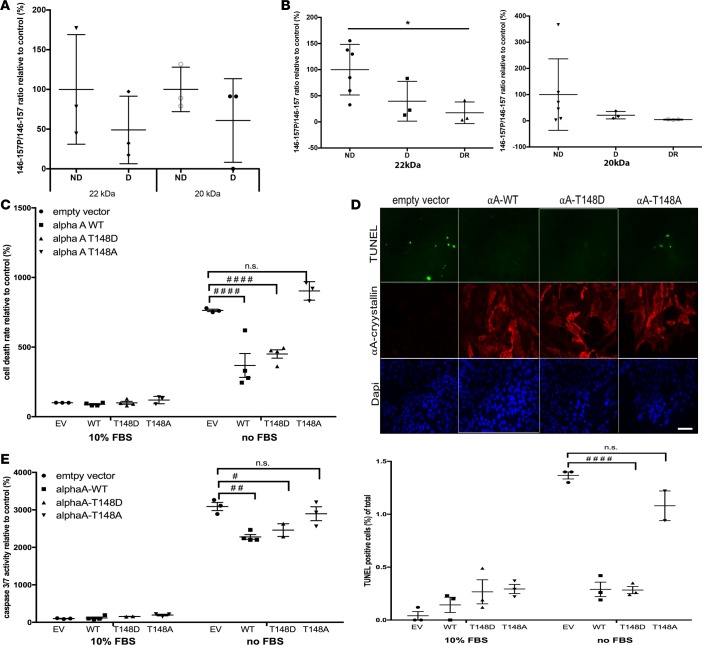
Figure 4. Phosphorylation on serine/threonine residue 148 of αA-crystallin, which is reduced by diabetes, regulates its neuroprotective function.
The 2 main αA-crystallin products identified by immunoblot (20 and 22 kDa) were immunoprecipitated from mouse and human retinal lysates and analyzed by mass spectrometry (A and B). The level of phosphorylation of the 146–157 peptide was analyzed by multiple reaction monitoring, and the ratio of phosphorylated to nonphosphorylated peptide is graphically represented for (A) 12-week diabetic (D, n = 3) or nondiabetic mice (ND, n = 3) and (B) nondiabetic (ND, n = 6), diabetic without retinopathy (D, n = 3), or diabetic with retinopathy human donors (DR, n = 3). *P ≤ 0.05, significantly different from nondiabetics. (C and E) Effect of αA-crystallin phosphorylation on the threonine residue 148 on retinal neuronal cell survival. Serum deprivation (no FBS) induced cell death was measured in neurons transfected with the empty vector (EV) versus neurons overexpressing WT (αA-WT), the phosphomimetic mutant (αA-T148D), or the nonphosphorylatable mutant (αA-T148A) of αA-crystallin on threonine 148. Cell death was assessed by DNA fragmentation ELISA (C), TUNEL staining (D), and caspase 3/7 activity assay (E). Representative immunofluorescence images of serum-deprived transfected neurons are shown for the TUNEL data (TUNEL in green, αA-crystallin in red, and nuclei in blue; scale bar: 50 μm), while graphic representation of the quantifications are shown for all 3 endpoints. #P ≤ 0.05, ##P ≤ 0.01, ####P ≤ 0.0001, significantly different from serum-starved EV-transfected cells. Each endpoint was measured on a minimum of 3 technical replicates in 3 independent experiments (2 for TUNEL). Statistical analysis was performed by 1-way ANOVA followed by Student-Newman-Keuls test.