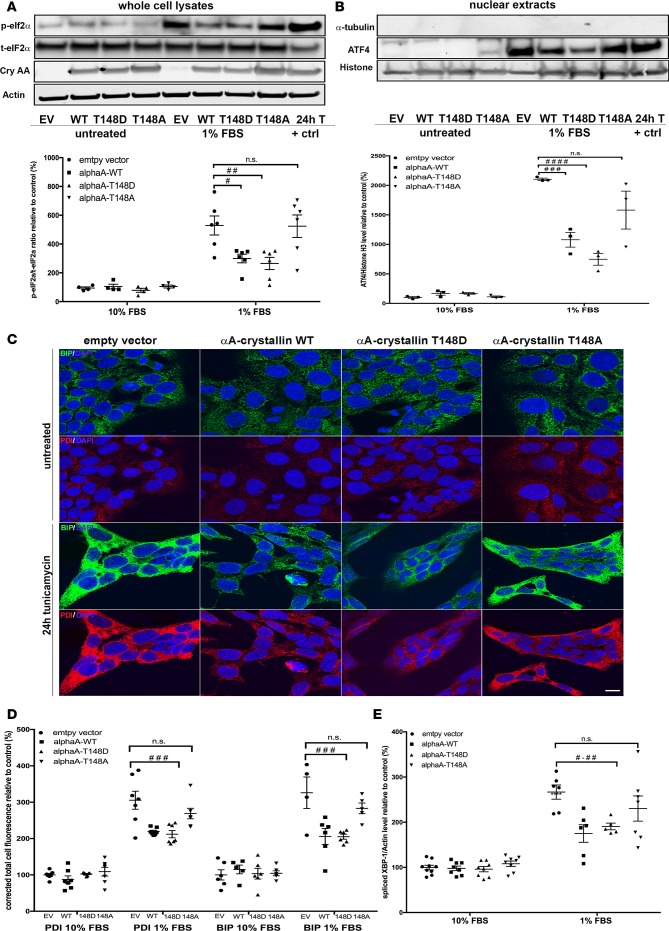
Figure 5. αA-crystallin attenuates endoplasmic reticulum stress in retinal neurons, an effect regulated by its phosphorylation on the 148 residue.
Retinal neurons were transfected with vectors overexpressing WT (αA-WT), the phosphomimetic mutant (αA-T148D), the nonphosphorylatable mutant (αA-T148A) of αA-crystallin on threonine 148, or the empty vector control (EV). The cells were then subjected to serum starvation (1% FBS) or tunicamycin (0.5 μg/ml, positive control), and the endoplasmic reticulum (ER) stress response was assessed by analysis of eukaryotic initiation factor α (eif2α) phosphorylation (A), activating transcription factor 4 (ATF4) nuclear translocation (B), binding immunoglobulin protein (BIP) and protein disulfide isomerase (PDI) induction (C and D), and X-box binding protein-1 (XBP-1) splicing (E). Representative images of the immunoblots and the corresponding graphic representation of the quantifications are shown for p-eIF2α, total-eIf2α, actin, and αA-crystallin (cryAA) (A) and ATF4, Histon H3, and α-tubulin (B). Representative images of the immunofluorescent staining obtained for BIP (green), PDI (red), and Hoechst (blue) (C) and the corresponding graphic representation of the relative quantification (D) (scale bar: 10 μm). Graphic representation of the quantitative real time PCR for spliced XBP-1 (E). Gene expression was normalized to the actin-encoding gene Actb. #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001, ####P ≤ 0.0001, significantly different from serum deprived EV-transfected cells. Each endpoint was measured on a minimum of 3 technical replicates in 3 independent experiments. Statistical analysis was performed by 1-way ANOVA followed by Student-Newman-Keuls test.