FIGURE 2.
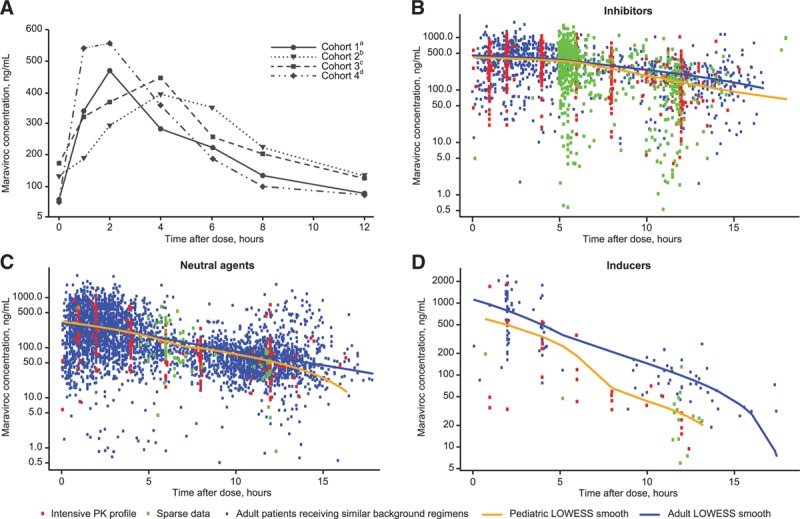
Maraviroc plasma concentration vs. time (A) at Week 2 by cohort; (B–D) by interaction category compared with adult data. A: Median Week 2 concentrations for Stage 1 participants enrolled in Stage 2 (N = 50; participants on final dose). B: Patients receiving maraviroc with potent CYP3A inhibitors. Data from pediatric patients (N = 85) were compared with data from adult patients (N = 125) receiving maraviroc at the approved dose of 150 mg BID in combination with lopinavir/ritonavir, darunavir/ritonavir or atazanavir/ritonavir in Studies A4001027, A4001028 (MOTIVATE 1 and 2),4 A400102911 and A4001098.12 C: Patients receiving maraviroc with neutral agents. Data from pediatric patients (N = 10) were compared with data from adult patients (N = 402) receiving maraviroc at the approved dose of 300 mg BID in combination with neutral agents in studies A4001026 (MERIT),3 A4001027, A4001028 (MOTIVATE 1 and 2),4 A400102911 and A4001098.12 D: Patients receiving maraviroc with potent CYP3A inducers. Data from pediatric patients (N = 2) were compared with data from adult patients (N = 27) receiving maraviroc at the approved dose of 600 mg BID in combination with efavirenz or etravirine in study A4001098.12aCohort 1: ≥2 to <6 years of age, maraviroc oral solution.bCohort 2: ≥6 to <12 years of age, maraviroc tablet formulation.cCohort 3: ≥6 to <12 years of age, maraviroc oral solution.dCohort 4: ≥12 to <18 years of age, maraviroc tablet formulation. BID indicates twice daily; LOWESS, locally weighted scatter plot smoothing.
