Figure 1.
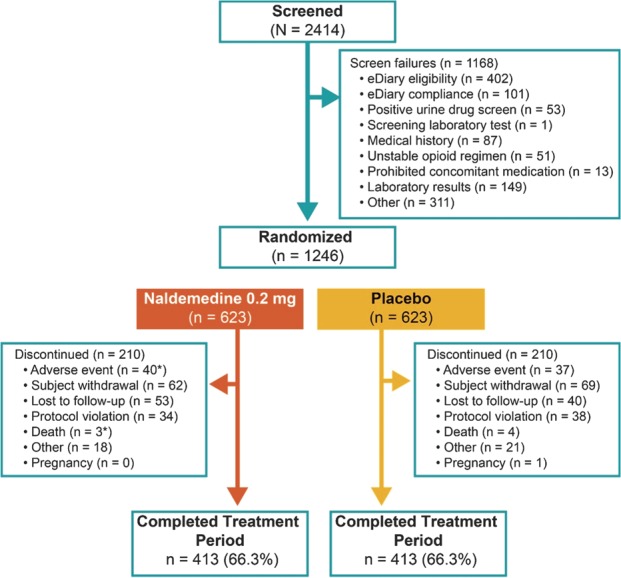
Patient disposition (CONSORT diagram). *One patient in the naldemedine group died 22 days after being removed from the study by the investigator because of an adverse event, which was recorded as the primary reason for discontinuation.

Patient disposition (CONSORT diagram). *One patient in the naldemedine group died 22 days after being removed from the study by the investigator because of an adverse event, which was recorded as the primary reason for discontinuation.