Figure 2.
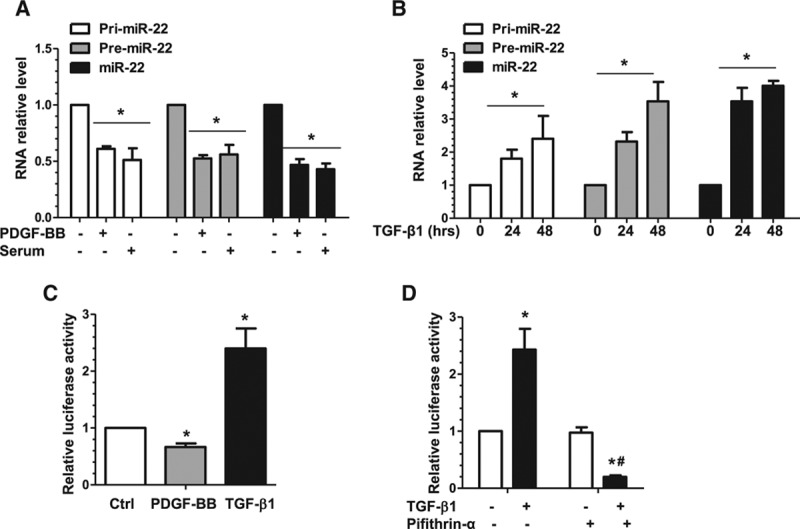
miR-22 is transcriptionally regulated in VSMC phenotypic modulation. A, PDGF-BB and serum significantly downregulated miR-22, and its primary (pri-miR-22) and precursor (premiR-22) transcripts, as well. Serum-starved VSMCs were used as the control (PDGF-BB–, Serum–), incubated with serum (20%), or incubated with PDGF-BB (10 ng/mL) for 3 hours. Total RNA was harvested and subjected to RT-qPCR analysis to examine miR-22 and its transcripts. B, TGF-β1 significantly upregulated miR-22, pri-miR-22, and premiR-22. RT-qPCR analysis was used to examine the miR levels in serum-starved VSMCs incubated with TGF-β1 (5 ng/mL) for 0, 24, and 48 hours. C, PDGF-BB significantly decreases, whereas TGF-β1 significantly increases miR-22 gene promoter activity. VSMCs transfected with miR-22 gene promoter (pGL3-miR-22) were subjected to serum starvation for 24 hours, followed by mock treatment (Ctrl) or incubation with PDGF-BB (10 ng/mL) or TGF-β1 (5 ng/mL) for 6 hours. Cell lysates were harvested and analyzed using the luciferase activity assay. D, TGF-β1 upregulated miR-22 in a P53-depedent manner. VSMCs transfected with miR-22 gene promoter (pGL3-miR-22) were subjected to serum starvation for 24 hours, followed by incubation with vehicle (TGF-β1–, Pifithrin-α–) or TGF-β1 (5 ng/mL) for 24 hours in the absence or presence of 15 µmol/L P53-specific inhibitor, Pifithrin-α. Cell lysates were harvested and subjected to the luciferase activity assay. Data and error bars in A through D represent the mean±SEM (n=3 in A, C, and D; n=4 in B). *P<0.05 (versus PDGF-BB–Serum– [A], 0 hours [B], Ctrl [C], or TGF-β1– [D]); #P<0.05 (versus TGF-β1+, Pifithrin-α–). miR-22 indicates microRNA-22; PDGF-BB, platelet-derived growth factor BB; RT-qPCR, reverse transcription quantitative polymerase chain reaction; TGF-β1, transforming growth factor β1; and VSMC, vascular smooth muscle cell.
