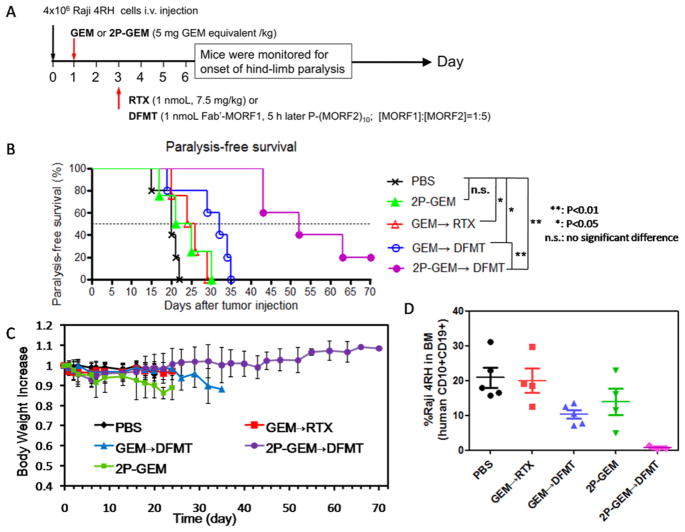
Figure 7.
In vivo validation of therapeutic efficacy. (A) Schedule of the treatments. (B) Paralysis-free survival and (C) body weight of mice after the treatments (n = 4–5). (D) Flow cytometry analysis of residual Raji 4RH cells (human CD10+CD19+) in the bone marrow (BM). BM cells were isolated from the femur of mice at end point (onset of paralysis), and Raji 4RH cells were dual stained with PE-labeled mouse antihuman CD10 and APC-labeled mouse antihuman CD19 antibodies. Three mice from 2P-GEM → DFMT treatment group that did not undergo paralysis were also sacrificed for antilymphoma efficacy comparison at days 27, 43, and 43, respectively.