Abstract
The authors explore five dimensions of research ethics: (1) normative ethics, which includes meta-ethical questions; (2) compliance with regulations, statutes, and institutional policies; (3) the rigor and reproducibility of science; (4) social value; and (5) workplace relationships. Each of the five dimensions is important not only because it addresses an aspect of good research done in a good manner, but also because it addresses the concerns of key stakeholders in the research enterprise. The five-dimension framework can guide institutions as they answer three questions central to any research ethics program: (1) Who should champion research ethics?, (2) What should interventions look like?, and (3) Who should participate in the interventions? The framework is valuable because the answers to these three questions are radically different depending upon the dimension under consideration. An expanded vision of research ethics does not entail that institutions should require additional online training or approvals from institutional review boards. However, without acknowledging all five dimensions, programs risk missing an important aspect of research ethics or ignoring the interests of important stakeholders.
Everyone agrees that research ethics is important. We propose, however, that not everyone agrees on a shared meaning of research ethics. Rather, they embrace divergent meanings that reflect their priorities, which arise from their personal needs, professional demands, or roles in society. The production of new knowledge—that is, the conduct of research—depends upon people who represent many different groups: compliance officers, educators, principal investigators (PIs), trainees, lab staff, citizens, legislators, journal editors and publishers, and funding agencies. In turn, each are stakeholders in research: in different ways, they each stand to benefit when research is conducted well and to lose when it is conducted badly.
In this Perspective, we explore five different dimensions of research ethics. We identified these dimensions through conceptual analysis of statements we have encountered across a cumulative 25 years of developing curricula for the responsible conduct of research (RCR),1–8 assessing RCR instruction programs and factors related to research integrity, 9–15 working with researchers who have had difficulties with research compliance,16,17 and serving clinical and translational science award (CTSA) programs that include cores in clinical research ethics, research design, and community engagement.18
We define research ethics as “doing good science in a good manner.” By “good science,” we mean science conducted according to common standards of excellence; for example, the criteria used by National Institutes of Health (NIH) scientific study sections:
the study addresses a significant topic with a premise supported by data; the study team is competent; the study involves novelty that advances the field; the approach utilizes rigorous methods; and the research environment provides appropriate resources.19
By “in a good manner” we mean all of the steps necessary to satisfy rules for RCR, including appropriate data storage, management of conflicts of interest, protection of human participants and animal subjects, proper lab safety, honest reporting of findings, proper citation of sources, and timely reporting of protocol deviations or serious adverse events to appropriate compliance offices.20,21 The need for a framework for research ethics arises in part from the fact that research ethics has been specified to the point of being disjointed. To illustrate, the office of research at our institution has 17 distinct compliance offices each corresponding to distinct federal requirements from animal care to export controls.
In conducting a conceptual analysis of the domains of research ethics based on stakeholder perspectives, we sought to identify dimensions that:
reflect an aspect of doing good research in a good manner;
are conceptually distinct, even if somewhat overlapping with other dimensions;
have distinct practical implications for determining champions of, ideal audiences for, and interventions to foster the dimension; and
are useful in organizing several more specific subtopics within the dimension (that is, the dimensions are “mid-level” concepts that are more specific than “research ethics” and more general than “informed consent”).
We argue that each of the five dimensions is important not only because each addresses an aspect of good research done in a good manner, but also because each addresses the concerns of key stakeholders in the research enterprise. Accordingly, researchers, research institutions, and other members of the community ought to dedicate appropriate time, effort, and concern to each dimension.
Five Dimensions of Research Ethics
We have identified five core dimensions of research ethics: (1) normative ethics, which includes meta-ethical questions; (2) compliance with regulations, statutes, and institutional policies; (3) the rigor and reproducibility of science; (4) social value; and (5) workplace relationships. Most dimensions are important to multiple groups; yet, in our experience, certain groups tend to express concerns about issues within a certain dimension more than other groups do. Below we describe each dimension and its groups of primary stakeholders. We illustrate some of the specific topics subsumed under each dimension through a series of questions commonly addressed when engaging the dimension.
Normative ethics
Normative ethics is ethics in the classic sense of determining what is right and wrong.22 Normative research ethics examines questions23 such as,
“Is informed consent necessary when patients are randomized to different, commonly accepted standards of care?”
“Is conducting harmful research on non-human animals that appear highly intelligent (e.g., chimpanzees and gorillas) ethical when the outcome may benefit humans?”
“Are some research questions (e.g., about the connection between genetics and traits such as criminality) forbidden if the consequences of the resulting knowledge could be socially harmful?”
Insofar as many different groups support research (or are complicit in research)—including not only researchers, but research institutions, funding agencies, taxpayers, publishers, and members of the public who consume the fruits of research—we are all stakeholders in normative ethics. Still, we believe that philosophers and policymakers tend to express concerns about normative ethical issues in research more than the average researcher working in a laboratory.
Compliance
Compliance consists of many different activities aimed at ensuring that those involved in actually conducting research (PIs, trainees, lab staff) follow federal research regulations, state laws, and institutional policies while they carry out their investigations.24 Compliance programs may address questions such as,
“Which training programs are mandatory and for whom?”
“When noncompliance—especially serious or persistent noncompliance—occurs, when does it need to be reported, to whom, and how?”
“What procedures can help a research team ensure that all informed consent forms have the proper stamp, date, and signature?”
Compliance rules often express commitment to an underlying value. For example, the value of participant self-determination underlies informed consent rules, and good stewardship of taxpayer dollars underlies rules about allowable costs on grants. Viewed from this perspective, everyone associated with research in some fashion has a stake in compliance. Nevertheless, different groups are primary stakeholders in different regards. Institutions and researchers frequently express concerns about administrative burdens and potential penalties; oversight bodies express concerns about noncompliance and their ability to enforce rules; and legislators express concerns when they receive reports of harms resulting from noncompliance. Researchers tend to express concerns particularly when compliance threatens other dimensions of research ethics, such as the timely conduct of rigorous research that could be of immediate social value.
Rigor and reproducibility
Rigor and reproducibility gets at the heart of what researchers typically mean by “good science.”25,26 Within the biomedical sciences, programs focused on enhancing the rigor and reproducibility of research ask questions such as,
“Has the study included animal subjects or human participants from both biological sexes to foster the generalizability of findings?”
“Have biological resources such as cell lines or antibodies been authenticated?”
“Have data been appropriately documented and deposited in a repository to enable replication studies?”
Researchers care deeply about such matters. Their work is motivated by the desire to discover truth and to develop new technologies—neither of which can occur when research is conducted in a sloppy manner. Because rigor and reproducibility largely constitute good science—the only kind of science that can offer social value and justify risks to subjects and financial investment in research27—everyone is a stakeholder in this dimension. Still, as a general rule, researchers, peer reviewers, and funding agencies express the greatest concern about scientific rigor and reproducibility as evidenced by new NIH guidelines on rigor and reproducibility for applicants and peer reviewers,25 editorials by publishers,26,28 and the growing body of literature by scientists on these matters.29–31
Social value
Social value means that research addresses problems of importance to society, generating knowledge used to solve real-world problems through new technologies or procedures.32–35 Those who seek to promote social value may ask,
“Does the study address a socially important topic?”
“Has the public been engaged to identify priorities, and have these priorities been truly considered in developing research questions?”
“Does the study include an appropriate follow-up or dissemination plan to ensure it has its intended impact?”
While scientists often view basic science as intrinsically valuable (and often of unforeseen practical value), this dimension acknowledges that science is rarely an individual, self-funded effort, and the priorities of stakeholders should have some influence on research priorities. Scientists have traditionally placed little emphasis on social value,36 yet its growing significance is readily apparent in several initiatives at the United States Department of Health and Human Services, including the amount of funding for the Patient-Centered Outcomes Research Institute37,38 and the requirement that all NIH-funded centers for clinical and translational science have community engagement components.18 Traditionally, groups that advocate improved health and education, congressmen and congresswomen who approve federal research budgets, and community-engaged researchers who support the needs of a population have all expressed the deepest concerns about this dimension. At times researchers have lamented the shift in focus at NIH and other agencies from basic, bench science to translational science.39,40
Workplace relationships
Workplace relationships—at least labeled as such—may be a newly identified aspect of research ethics. Although researchers may not have considered this dimension much previously, we believe it is integral to doing good research in a good manner. The specific form that respectful and effective workplace relationships take may vary significantly across cultures with different hierarchies and different workload expectations. Still, we submit that every culture harbors those who are good to work with (and for), and those who are not. The workplace relationships dimension of research ethics entails questions such as,
“Do members of the research team welcome diversity and treat one another with respect?”
“Does the PI set workloads and deadlines reasonably to enable work to be done without cutting corners or compromising quality?”
“Does open communication enable team members to express concerns about the quality of work or the work environment”
In the broadest sense, everyone is a stakeholder in workplace relationships because when relationships turn sour, the risk of poor work performance, staff turnover, and even intentional sabotage increases, which can, in turn, threaten the quality of science41–43; nevertheless, the most obvious stakeholders in this dimension are researchers themselves—PIs, graduate students, post-docs, and research or lab staff. Typically, research staff and trainees—those who work full-time in research labs and teams—have less power than PIs and express the greatest concerns about workplace relationships. Of course, workplace relationships also include researchers’ relationships with compliance officers, grant administration staff, journals, and even funding agencies—as well as those stakeholders’ relationships with one another.
Intersection of Dimensions and Potential Conflicts
While we have presented the five dimensions as distinct, each is related to other domains, often so intimately that researchers cannot fulfill obligations in one dimension without fulfilling at least some basic obligations in others; however, at other times, the dimensions may conflict. We illustrate below how dimensions may overlap or conflict by examining the question of informed consent in pragmatic cluster randomized trials.
Consider a trial that randomizes primary care provider groups to receive an educational reminder and feedback on practices (following review of patient records) to promote adherence to radiology guidelines for ordering lumbar spine x-rays.44 Such studies are often praised for their social value: they have the ability to affect real-world medical practices, improving patient care while reducing costs. Yet, in order to achieve their aims, they must recruit entire practices of physicians in sufficient numbers to generate adequate statistical power. From a compliance perspective, the question of informed consent in pragmatic trials has been debated.45 Is the consent of individual physicians within a practice required? Is the consent of patients required if the study will involve patient record review or if it might affect their treatment? The answer to these questions will affect scientific rigor and reproducibility. If physicians within a practice can opt out, then the effects of the intervention may be confounded by opt-out rates within practices; and if physicians know the study focuses on radiology guidelines, then even those in a control group may read the guidelines, again confounding the effects of the intervention. The question of consent may also be treated as a question of workplace relationships. Can one representative of a physician practice speak on behalf of all physicians in the practice? If the physicians have not been consulted, how will this affect team morale when study results are published? How might patient trust be affected if the patients themselves are not engaged in such studies? If questions of consent are treated not only as a matter of compliance, but one of normative ethics, then all of the proceeding considerations may come into play as diverse principles and values are balanced.46,47
Recognizing the five dimensions of research ethics will not resolve conflicts between them; however, considering these dimensions might change the nature of the debates. Instead of viewing conflicts between compliance and research rigor, for example, as a conflict between research ethics and good science, researchers may realize that the conflict actually represents two dimensions of the complexity that is research ethics. This realization adds weight to the need both to consider all dimensions seriously and to advocate regulatory reform or institutional change when appropriate. Similarly, in the world of financial conflicts of interest in research—for example, those arising from the sponsorship of clinical trials by industry—researchers may acknowledge not only the need to avoid bias due to financial interests (representing the dimensions of compliance and rigor and reproducibility), but also the need to innovate and disseminate (the dimension of social value). Treating both needs as a matter of research ethics adds gravitas to the discussion, and might inspire parties to seek novel approaches to managing bias and risks.48
Are We Missing the Most Important Elements?
In his seminal 1966 article, “Ethics and Clinical Research,”49 Henry Beecher referred to “the presence of an intelligent, informed, conscientious, compassionate, responsible investigator” as the most reliable safeguard in clinical research. Likewise, the International Conference on Harmonisation’s guidelines for clinical investigators hold individual investigators responsible for ensuring compliance, obtaining informed consent, and protecting the rights and welfare of participants.50 Readers might then ask why we have not included the integrity of researchers as a core dimension. Similarly, the matter of organizational climate of research integrity has received increasing attention. A new measure of an organization’s research integrity climate—which includes dimensions such as regulatory support and research integrity socialization—has been used to demonstrate a relationship between institutional climate and self-reports of more positive researcher behaviors.51 In fact, when the U.S. Research Council addressed research integrity in its seminal 2002 report,52 it focused explicitly on the integrity of individual researchers and the integrity of research environments, arguing that these are the two pillars of ethical research.
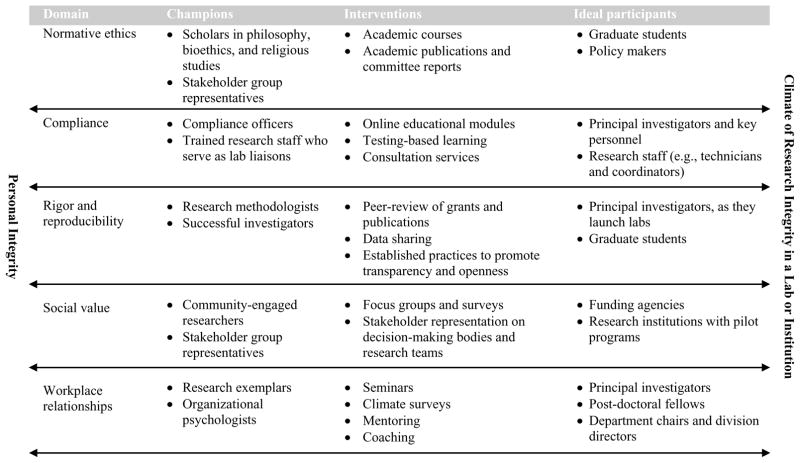
Our framework supports and further elucidates these emphases on individual and organizational research integrity. From the earliest days of discussion of virtue53 through modern discussion of the formation of moral identity and character,54 philosophers and educators have seen that integrity is reflexive: We attribute good actions to integrity, yet good actions are also constitutive of integrity. Put another way: it is not possible to maintain personal integrity or a climate of integrity without demonstrating it through ethical behavior—and without personal integrity and a climate of integrity, ethical behavior will not be consistent. Our five dimensions define how personal integrity and a climate of research integrity at all levels—the lab, the department, and the organization—are both constituted and expressed. Thus, personal integrity and climate of research integrity appear as bookends with two-way arrows in Chart 1, illustrating that they are necessary for and derived from the five dimensions.
Chart 1.
Promoting the Five Dimensions of Research Ethics to Generate a Climate of Research Integrity
Implications
Any simple framework for understanding something as socially complex as ethics in research is necessarily incomplete; however, such a framework does offer value if it is useful in guiding decision-making and action. Our framework of five research ethics dimensions can guide institutions as they answer three questions central to any research ethics program: (1) Who should champion research ethics?, (2) What should interventions look like?, and (3) Who should participate in the interventions? The framework is valuable because the answers to these three questions are radically different depending upon the dimension under consideration. Chart 1 presents an overview of how one might answer the three questions across each of the five dimensions. In what follows, we highlight a few key points.
Who should champion each dimension of research ethics?
When thinking of research ethics, members of the scientific research community may most naturally think of ethicists and philosophers as the relevant experts. This makes sense if research ethics represents only normative ethics; however, in the dimension of compliance, research integrity officers and compliance committee members generally have greater expertise. Research methodologists and successful investigators with significant peer review experience may be in the best position to promote rigor and reproducibility in research. Directors of health agencies and community-engaged researchers may have an excellent sense of social value, particularly of community problems that research might address. Researchers who are known for their research skill and personal integrity (so-called exemplars) and organizational psychologists may be best positioned to provide leadership on workplace relationships. Once viewed through the lens of the five dimensions, a greater number of potential experts, leaders, and champions of research ethics emerge, and the inadequacy of delegating research ethics to an ethicist becomes immediately apparent.
What should interventions to foster each dimension of research ethics look like, and who should participate in those interventions?
Online learning and formal face-to-face training may have a place in the transfer of knowledge about compliance with federal regulations and institutional expectations; however, other dimensions require fundamentally different approaches. Rigor and reproducibility can be promoted by promulgating transparency and openness29 among journal editors, data repositories, and peer reviewers. Social value can be promoted by engaging communities—through, for example, focus groups, surveys, or representation on committees—and broadly disseminating results to those who determine what research gets funded (peer reviewers and funding programs). Positive workplace relationships can be fostered by institutional leaders in diverse ways: recognizing exemplary mentors, providing leadership training workshops, and publicizing best practices for constructive communication, including how to hold effective meetings, how to support diversity and inclusion, and how to be approachable to (and protect) those who express workplace concerns. Normative ethics can be engaged through courses that include case study discussion and the application of basic principles, values, or virtues to problems. Once the five dimensions of research ethics are recognized, the inadequacy of providing key personnel with online learning modules and trainees with 8 hours of didactic instruction becomes apparent.
Conclusions
The academic research community needs a broader concept of research ethics experts: People who lack expertise in one dimension may be the ideal leaders or champions in another dimension. The research community also needs a broader concept of who should participate in interventions; for example, a seasoned PI may need support in managing compliance, personnel, or data from multiple sites (and may not know that such support is needed), while an institutional research compliance officer may need a briefing on how community members actually view alleged risks in research. Finally, the research community must realize that research ethics requires institutions to transcend themselves, to engage communities, policymakers, and funding agencies to ensure that research abides by genuinely ethical principles and serves the needs of society. These dimensions may conflict with one another; when they conflict, processes should be established for resolving conflicts while acknowledging that each of the dimensions is essential in an ethical research climate.
Notably, in identifying and describing five dimensions of research ethics, we are not suggesting that trainees or investigators need more online training or further institutional review board approvals. Rather, we believe the research community needs leaders with a broad vision of research ethics—leaders who recognize that fostering research ethics cannot be delegated to any one individual or even one group of stakeholders. As we illustrate in Chart 1, a climate of research integrity results from a visible commitment to the priorities of diverse stakeholders in the research enterprise; once established, such a climate sustains ethics in all five dimensions. Personal integrity operates in a similar manner: Personal integrity is needed to engage each of these dimensions successfully, yet engaging them successfully is also partially constitutive of the integrity of researchers.
Considering how much an academic health center spends—in terms of both money and time—on research compliance each year, it is not outrageous to suggest that a research institution’s chief executive officer (CEO) might convene a multi-stakeholder research ethics committee including appropriate leaders who, collectively, have expertise in all five dimensions. Such a committee could design a plan addressing each area in Chart 1, track progress, and report annually to the CEO. In principle, this approach could lead to greater efficiency by playing to existing but untapped expertise while reducing gaps in research ethics programs. To be clear, not all endeavors need to be framed or marketed as research ethics. Some of the dimensions address matters of human resources, economic impact, or scientific skill; if audiences are more receptive to research ethics activities when framed in these ways, then such alternate frames may be beneficial. Still, recognizing all of these dimensions as aspects of an institution’s climate of research integrity ensures that each of us will take into consideration the many dimensions of ethics integral to the research enterprise.
Acknowledgments
Funding/Support: This work was supported by the National Center for Advancing Translational Science Clinical and Translational Science Award to Washington University in St. Louis, UL1 TR000448. The effort of A.L. Antes was supported in part by the National Human Genome Research Institute, K01HG008990.
Footnotes
Other disclosures: None reported.
Ethical approval: Reported as not applicable.
Previous presentations: A version of this paper was presented at the 2016 meeting of the American Society for Bioethics and Humanities in Washington DC.
Contributor Information
James M. DuBois, Steven J. Bander Professor of Medical Ethics and Professionalism, and he is director, Center for Clinical and Research Ethics, Washington University School of Medicine, St. Louis, Missouri.
Alison L. Antes, Assistant professor of medicine and assistant director, Center for Clinical and Research Ethics, Washington University School of Medicine, St. Louis, Missouri.
References
- 1.Antes AL. A systematic approach to instruction in research ethics. Accountability in Research: Policies and Quality Assurance. 2014;21:50–67. doi: 10.1080/08989621.2013.822269. [DOI] [PubMed] [Google Scholar]
- 2.Antes AL, Thiel CE, Martin LE, et al. Applying cases to solve ethical problems: The significance of positive and process-oriented reflection. Ethics Behav. 2012;22:113–130. doi: 10.1080/10508422.2012.655646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mumford MD, Connelly S, Brown RP, et al. A sensemaking approach to ethics training for scientists: Preliminary evidence of training effectiveness. Ethics Behav. 2008;18:315–339. doi: 10.1080/10508420802487815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DuBois JM, Dueker JM. Teaching and assessing the responsible conduct of research: A Delphi consensus panel report. Journal of Research Administration. 2009;40:49–70. [PMC free article] [PubMed] [Google Scholar]
- 5.DuBois JM, Dueker JM, Anderson EE, Campbell J. The development and assessment of an NIH-funded research ethics training program. Acad Med. 2008;83:596–603. doi: 10.1097/ACM.0b013e3181723095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuBois JM, Schilling DA, Heitman E, Steneck NH, Kon A. Instruction in the responsible conduct of research: An inventory of programs and materials within CTSAs. Clinical and Translational Science. 2010;3:109–111. doi: 10.1111/j.1752-8062.2010.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuBois JM, editor. RCR Casebook: Stories Worth Discussing. Rockville, MD: U.S. Department of Health and Human Services Office of Research Integrity; 2013. [Accessed August 16, 2017]. http://ori.hhs.gov/rcr-casebook-stories-about-researchers-worth-discussing. [Google Scholar]
- 8.DuBois JM, Ciesla JE, Voss K. Research ethics in US medical education: An analysis of ethics course syllabi. Investigating Research Integrity, Proceedings of the First ORI Research Conference on Research Integrity; Bethesda, Maryland: Department of Health and Human Services Office of Research Integrity; 2002. [Accessed August 16, 2017]. https://ori.hhs.gov/documents/proceedings_rri.pdf. [Google Scholar]
- 9.Antes AL, Brown RP, Murphy ST, et al. Personality and ethical decision-making in research: The role of perceptions of self and others. Journal of empirical research on human research ethics. 2007;2:15–34. doi: 10.1525/jer.2007.2.4.15. [DOI] [PubMed] [Google Scholar]
- 10.Antes AL, Wang X, Mumford MD, Brown RP, Connelly S, Devenport LD. Evaluating the effects that existing instruction on responsible conduct of research has on ethical decision making. Acad Med. 2010;85:519–526. doi: 10.1097/ACM.0b013e3181cd1cc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antes AL, DuBois JM. Aligning objectives and assessment in responsible conduct of research instruction. Journal of Microbiology & Biology Education. 2014;15:108–116. doi: 10.1128/jmbe.v15i2.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antes AL, Chibnall JT, Baldwin KA, Tait RC, Vander Wal JS, DuBois JM. Making professional decisions in research: Measurement and key predictors. Account Res. 2016;23:288–308. doi: 10.1080/08989621.2016.1171149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DuBois JM, Chibnall JT, Tait RC, et al. Professional Decision-Making in Research (PDR): The Validity of a New Measure. Science and Engineering Ethics. 2016;22:391–416. doi: 10.1007/s11948-015-9667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DuBois JM, Chibnall JT, Gibbs J. Compliance Disengagement in Research: Development and Validation of a New Measure. Science and engineering ethics. 2016;22:965–988. doi: 10.1007/s11948-015-9681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DuBois JM, Anderson EE, Chibnall J, et al. Understanding research misconduct: A comparative analysis of 120 cases of professional wrongdoing. Account Res. 2013;20:320–338. doi: 10.1080/08989621.2013.822248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuBois JM, Anderson EE, Chibnall J. Assessing the need for a research ethics remediation program. Clinical and Translational Science. 2013;6:209–213. doi: 10.1111/cts.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DuBois JM, Chibnall JT, Tait RC, Vander Wal JS. Misconduct: Lessons from researcher rehab. Nature. 2016;534:173–175. doi: 10.1038/534173a. [DOI] [PubMed] [Google Scholar]
- 18.Institute of Medicine. The CTSA Program at NIH: Opportunities for Advancing Clinical and Translational Research. Washington, D.C: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 19.National Institutes of Health Office of Extramural Research. [Accessed August 16, 2017];Definitions of Criteria and Considerations for Research Project Grant (RPG/X01/R01/R03/R21/R33/R34) Critiques. 2016 Mar 21; https://grants.nih.gov/grants/peer/critiques/rpg_D.htm.
- 20.Shamoo AE, Resnik DB. Responsible Conduct of Research. 3. New York: Oxford University Press; 2015. [Google Scholar]
- 21.National Academies of Science. On Being a Scientist: A Guide to Responsible Conduct in Research. 3. Washington DC: National Academics Press; 2009. [PubMed] [Google Scholar]
- 22.Pojman LP, Fieser J. Ethics: Discovering Right and Wrong. 8. Boston: Wadsworth Publishing; 2016. [Google Scholar]
- 23.Comstock G. Research Ethics: A Philosophical Guide to the Responsible Conduct of Research. New York: Cambridge University Press; 2013. [Google Scholar]
- 24.Dade A, Olafson L, DiBella SM. Implementing a Comprehensive Research Compliance Program: A Handbook for Research Officers. Charlotte, NC: Information Age Publishing, Inc; 2016. [Google Scholar]
- 25.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505:612–613. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcus E. Credibility and reproducibility. Cell. 159:965–966. doi: 10.1016/j.cell.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283:2701–2711. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 28.McNutt M. Journals unite for reproducibility. Science. 2014;346:679. doi: 10.1126/science.aaa1724. [DOI] [PubMed] [Google Scholar]
- 29.Nosek BA, Alter G, Banks GC, et al. Scientific standards. Promoting an open research culture. Science. 2015;348:1422–1425. doi: 10.1126/science.aab2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nosek B. Psychology: Estimating the reproducibility of psychological science. Science. 2015;349(6251) doi: 10.1126/science.aac4716. [DOI] [PubMed] [Google Scholar]
- 31.Freedman LP, Cockburn IM, Simcoe TS. The economics of reproducibility in preclinical research. PLoS biology. 2015;13:e1002165. doi: 10.1371/journal.pbio.1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazelkorn E. The Chronicle of Higher Education. Aug 15, 2012. Measuring value: Societal benefits of research. [Google Scholar]
- 33.Jones B, Lightfoot A, De Marco M, et al. Community-responsive research priorities: Health research infrastructure. Prog Comm Hlth Partn. 2012;6:339–348. doi: 10.1353/cpr.2012.0045. [DOI] [PubMed] [Google Scholar]
- 34.Chen PG, Diaz N, Lucas G, Rosenthal MS. Dissemination of results in community-based participatory research. American Journal of Preventive Medicine. 2010;39:372–378. doi: 10.1016/j.amepre.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 35.McBride T, Coburn A, Mackinney C, Mueller K, Slifkin R, Wakefield M. Bridging health research and policy: Effective dissemination strategies. Journal of Public Health Management and Practice. 2008;14:150–154. doi: 10.1097/01.PHH.0000311893.80701.7a. [DOI] [PubMed] [Google Scholar]
- 36.Nowotny H, Scott P, Gibbons M. Re-Thinking Science: Knowledge and the Public in an Age of Uncertainty. Cambridge, UK: Polity; 2001. [Google Scholar]
- 37.Patient-Centered Outcomes Research Institute (PCORI) [Accessed August 22, 2017];Research & Results, Patient-Centered Outcomes Research. Updated November 2013. http://www.pcori.org/research-results/patient-centered-outcomes-research.
- 38.Patient-Centered Outcomes Research Institute (PCORI) [Accessed August 16, 2017];Funding Opportunities. 2017 http://www.pcori.org/funding-opportunities.
- 39.Hörig H, Marincola E, Marincola FM. Obstacles and opportunities in translational research. Nature Medicine. 2005;11:705–708. doi: 10.1038/nm0705-705. [DOI] [PubMed] [Google Scholar]
- 40.Hobin JA, Deschamps AM, Bockman R, et al. Engaging basic scientists in translational research: Identifying opportunities, overcoming obstacles. J Transl Med. 2012;10:72. doi: 10.1186/1479-5876-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen CCSL. Lab dynamics: Management and Leadership Skills for Scientists. 2. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2012. [Google Scholar]
- 42.Macrina FL. Dynamic Issues in Scientific Integrity: Collaborative Research. American Academy of Microbiology; [Accessed August 16, 2017]. https://www.asm.org/images/stories/documents/dynamicissuesinscientificintegrity.pdf. [PubMed] [Google Scholar]
- 43.Adams LG. Putting together a scientific team: Collaborative science. Trends in Microbiology. 2014;22:483–485. doi: 10.1016/j.tim.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Hutton JL, Eccles MP, Grimshaw JM. Ethical issues in implementation research: A discussion of the problems in achieving informed consent. Implement Sci. 2008;3:52. doi: 10.1186/1748-5908-3-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menikoff J. The Unbelievable Rightness of Being in Clinical Trials. Hastings Cent Rep. 2013;43:S30–S31. doi: 10.1002/hast.136. [DOI] [PubMed] [Google Scholar]
- 46.Gopichandran V, Luyckx VA, Biller-Andorno N, et al. Developing the ethics of implementation research in health. Implement Sci. 2016;11:161. doi: 10.1186/s13012-016-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Childress JF, Faden RR, Gaare RD, et al. Public health ethics: mapping the terrain. The Journal of Law, Medicine & Ethics. 2002;30:170–178. doi: 10.1111/j.1748-720x.2002.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 48.Association of American Medical Colleges-Association of American Universities. Protecting patients, preserving integrity, advancing health: Accelerating the implementation of COI policies in human subjects research. Washington DC: AAMC-AAU; Feb, 2008. [August 16, 2017]. https://members.aamc.org/eweb/upload/protecting%20Patients,%20Preserving%20Integrity.pdf. [Google Scholar]
- 49.Beecher HK. Ethics and clinical research. The New England Journal of Medicine. 1966;274:1354–1360. doi: 10.1056/NEJM196606162742405. [DOI] [PubMed] [Google Scholar]
- 50.International Conference on Harmonisation. Good Clinical Practice (ICH GCP) [Accessed August 22, 2017];4 INVESTIGATOR. 2016 http://ichgcp.net/4-investigator.
- 51.Crain AL, Martinson B, Thrush C. Relationships between the Survey of Organizational Research Climate (SORC) and self-reported research practices. Science and Engineering Ethics. 2013;19:835–850. doi: 10.1007/s11948-012-9409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Institute of Medicine National Research Council. Integrity in Scientific Research: Creating an Environment That Promotes Responsible Conduct. Washington, DC: National Academies Press; 2002. [PubMed] [Google Scholar]
- 53.Aristotle . In: The Nicomachean ethics. Ross D, translator. Oxford: Oxford University Press; 1980. [Google Scholar]
- 54.Gibbs JC. Moral Development and Reality: Beyond the Theories of Kohlberg, Hoffman, and Haidt. 3. New York: Oxford University Press; 2013. [Google Scholar]