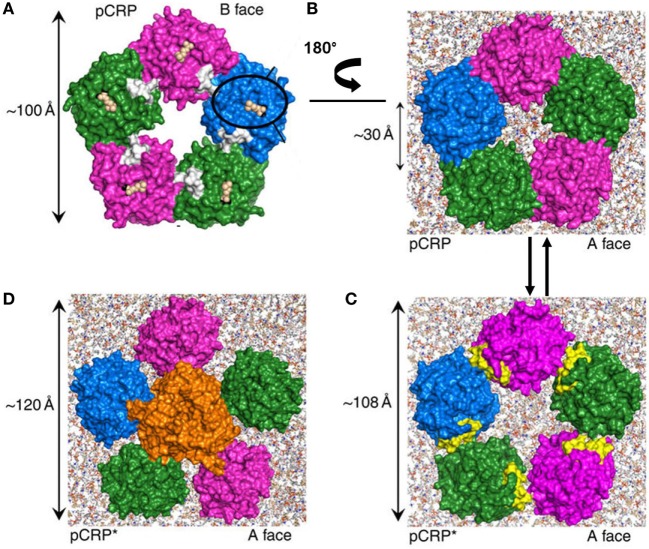
Figure 1.
Proposed model of the conversion from the strained to the relaxed conformations of pentameric C-reactive protein (CRP). (A) Solid surface representation of the crystal structure of human CRP in its “strained” conformation (pCRP) bound to phosphocholine (PCh) (PDB entry 1B09) (54). The view shown is from the membrane binding “B face” of pCRP. The individual subunits are represented color-coded, with PCh (cream spheres) and Ca2+ ions (black spheres) occupying the ligand binding site on each subunit. (B) Modeled interaction of pCRP with a phospholipid bilayer. View is from “above,” looking down onto the pCRP “A face.” Each pCRP subunit can independently bind to a PCh head group of the bilayer. Exposure to lysoPCh triggers reversible conversion of pCRP to pCRP*. (C) Pentameric pCRP*, same view as in (B). As the individual CRP subunits move apart, a neoepitope (colored yellow) is exposed. (D) The globular head of C1q inserts itself into the inner annular void of pCRP* forcing the subunits further apart [adapted from Braig et al. (68)].