FIGURE 3.
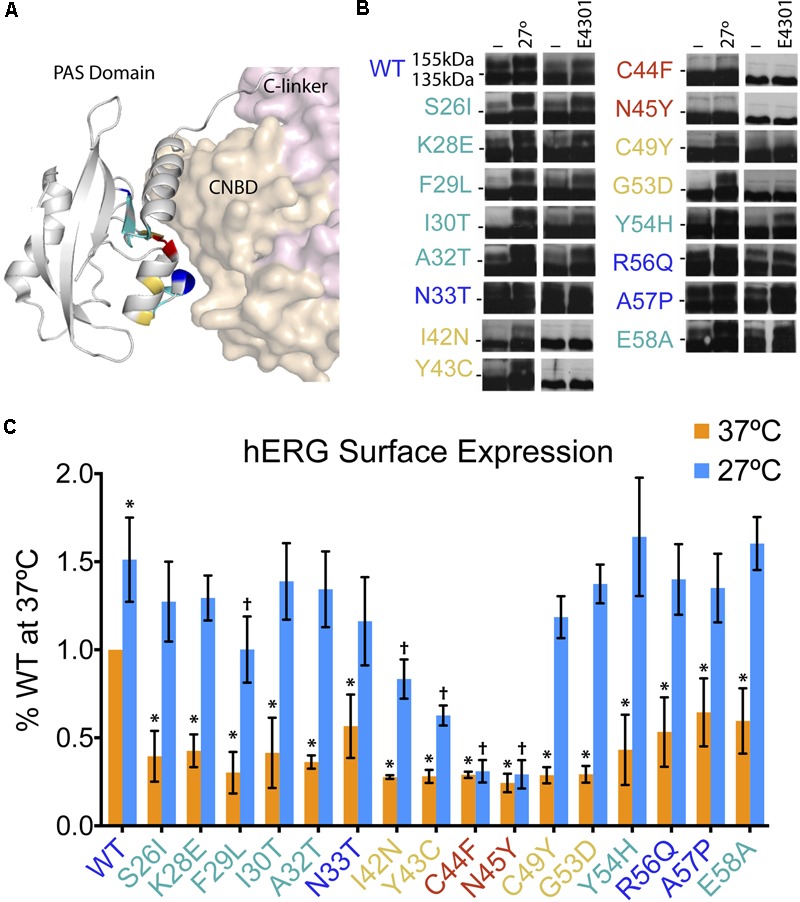
Low temperature rescue of defective surface trafficking in 16 LQT2 hERG channels with mutations in the PAS domain. (A) Structure of the hERG PAS domain in complex with CNBD, adapted from Wang and MacKinnon (2017) (PDB: 5VA2). Positions of 16 LQT2 mutations highlighted according to their reported impact on hERG trafficking as assessed by the prevalence of the 155-kDa mature protein and the rescue of this band by low temperature and/or E4031–WT glycosylation (blue), uncorrectable (red), temperature correctable only (yellow), and temperature and E4031 correctable (teal). (B) Western blot analyses of PAS domain LQT2 mutants reproduced from Anderson et al. (2014) (with permission from Nature Communications). Horizontal dashes at the sides of the blots represent 140 kDa. (C) Quantification of surface hERG channels (Alexa647 fluorescence) from flow cytometry experiments (n = 8878–30497 cells; N = 4) for WT and LQT2 mutant hERG channels at 37 and 27°C. Data are normalized to WT hERG surface expression at 37°C. ∗p < 0.02 versus WT 37°C, †p < 0.02 versus WT 27°C, two-way ANOVA followed by Dunnett’s test.
