Figure 6.
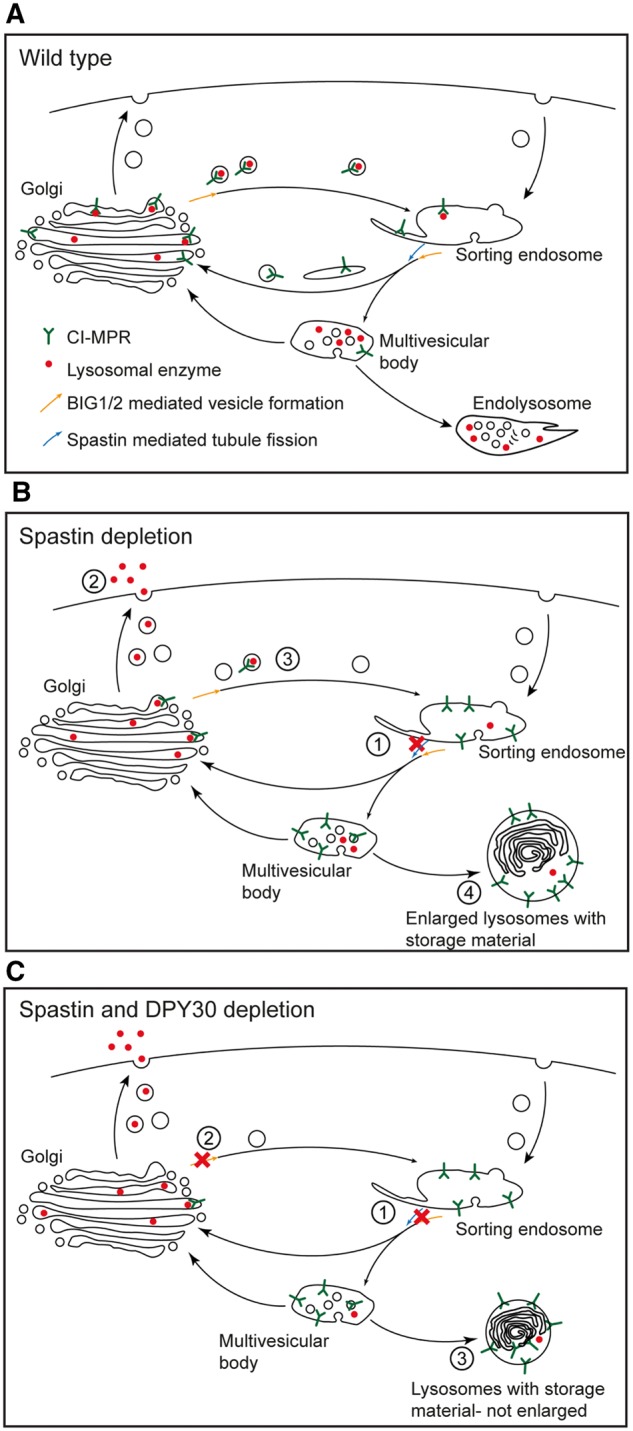
Model of role of spastin and DPY30 in membrane traffic pathways, and how these might influence lysosomes when disrupted. (A) In the normal situation, M6PR cycles between endosomes and the Golgi, capturing lysosomal enzymes at the Golgi for delivery back to the endolysosomal system. The sorting endosome matures into the multivesicular body and the accompanying acidification promotes dissociation of lysosomal enzymes from M6PR. During the maturation process, empty M6PR is sorted back to the Golgi in endosomal tubules that require spastin, DPY30 and likely BIG1/2 to break efficiently from the parent endosome. (B) In cells lacking spastin endosomal tubule fission is deficient (1), so M6PR traffic from the endosome-to-Golgi is inhibited and M6PR accumulates in the endolysosomal pathway. Lack of M6PR at the Golgi means that lysosomal enzymes are not captured, and instead are secreted (2) rather than delivered efficiently to the endolysosomal system (3). These effects result in the development of enlarged (through failure of membrane sorting away from the lysosomal pathway and substrate accumulation), morphologically abnormal (through substrate accumulation) lysosomes (4). (C) In cells lacking spastin and DPY30, traffic of M6PR from the endosomes to the Golgi is inhibited by inefficient endosomal tubule fission (1), but in addition DPY30 promotes BIG1/2-mediated traffic out of the Golgi, so lack of DPY30 inhibits Golgi to endosome traffic (2). Both effects reduce enzyme delivery to the lysosome, but will tend to have opposing impacts on lysosomal size. Thus in cells lacking both spastin and DPY30, lysosomal size depends on the balance between these two effects, but effects on enzyme delivery could be additive (3), especially in the case of haploinsufficiency of both.
