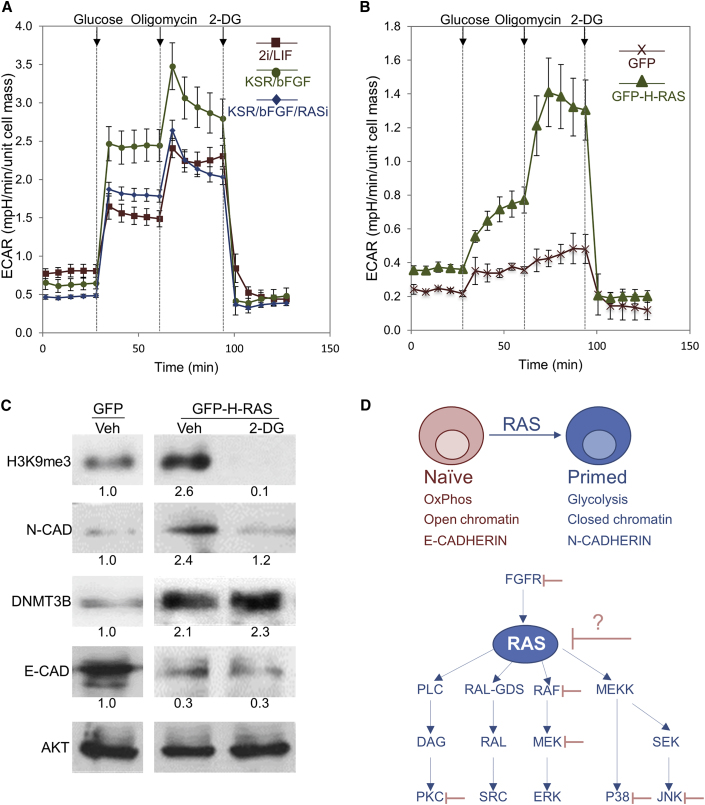
Figure 6.
RAS Induces Glycolysis in mESCs
(A) mESCs were grown in the indicated media (2i/LIF or KSR/bFGF) or in KSR/bFGF in the presence of RASi (75 μM) or control (Veh). Extracellular acidification rate (ECAR) was recorded before and following the addition of glucose, oligomycin (inhibitor of ATP synthase which blocks OxPhos), or 2-deoxyglucose (2-DG, inhibitor of glycolysis).
(B) mESCs were grown in 2i/LIF conditions and transfected with a plasmid encoding for GFP fused to H-RAS (GFP-H-RAS) or GFP alone. ECAR was recorded before and following the addition of glucose, oligomycin, or 2-DG.
(C) mESCs were grown in 2i/LIF conditions and transfected with a plasmid encoding for GFP fused to H-RAS (GFP-H-RAS) or GFP as control. On the next day, 2-DG or Veh was added, and 48 hr later, cells were lysed and subjected to western blot analysis of the indicated proteins. AKT served as loading control. Values represent densitometry analysis of two independent experiments.
(D) Upper panel: schematic illustration of RAS function in the transition to the primed state. Lower panel: selected signaling pathways that are regulated by RAS, among them, several factors (FGFR, PKC, RAF, MEK, P38, and JNK) that are positioned upstream or downstream of Ras signaling pathway were targeted by pharmacological inhibitors to stabilize human cells in the naive state. We propose that, since RAS controls a large set of crucial signaling protein, its targeting may be useful approach for reprogramming human cells into naive state. Data shown are mean ± SD from three independent experiments.