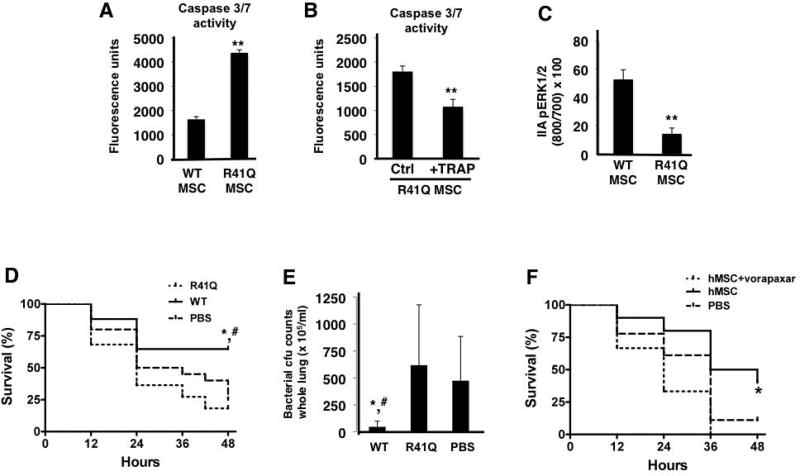
Figure 5. R41Q-PAR1 mutated MSCs demonstrate increased cell death when exposed to inflammatory stimuli in vitro and no therapeutic capacity in vivo.
When exposed to TNF-α and cycloheximide, R41Q-PAR1 mutated MSCs demonstrated increased cell death compared to WT MSCs as measured by caspase 3/7 activity (A, **p < 0.01 for R41Q MSCs vs WT MSCs, n = 6 per group). Pre-treatment of R41Q-PAR1 mutated MSCs with 10 µM of TRAP for 1 h reduced the quantity of cell death upon exposure to TNF-α and cycloheximide (B, **p < 0.01 for R41Q MSC + TRAP vs R41Q MSC, n = 6 per group). R41Q-PAR1 mutated MSCs exhibited significantly less ERK1/2 phosphorylation than WT MSCs after 5 min of 5 nM of thrombin stimulation (C, **p < 0.01 for R41Q MSC vs WT MSC, n = 3 per group). In vivo, R41Q-PAR1 mutated MSCs showed no evidence of therapeutic benefit in terms of 48 h survival or whole lung bacterial burden (D, E, *, # p < 0.05 for WT MSCs vs PBS and R41Q MSCs, respectively, n = 17–22 per group). Human MSCs were incubated with vorapaxar at 0.3 µM for 30 min and then administered as a treatment in the in vivo model. Vorapaxar pre-incubation significantly inhibited the therapeutic capacity of human MSCs in vivo when compared to non-pre-treated cells (F, *p < 0.05 for hMSCs vs hMSCs + vorapaxar, n = 10–18 per group).