FIGURE 5. EBP1 displays reduced binding to DPPA4 upon differentiation of NT2 cells.
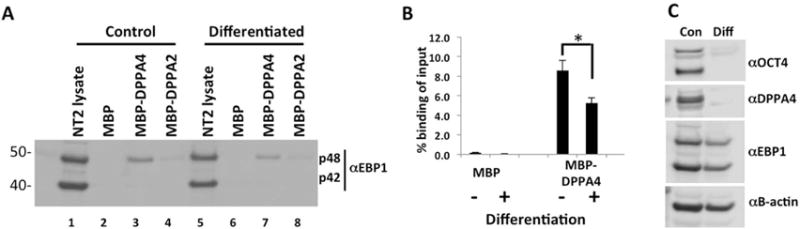
(A) Whole cell lysates of NT2 cells differentiated for 8 days with all-trans retinoic acid (at-RA) were incubated with amylose-bound MBP-DPPA4 or MBP-DPPA2, unbound proteins were washed, and interacting protein was detected through western blotting with anti-EBP1. Undifferentiated whole cell lysates were included as a control. Western blot is representative of three independent biological replicates. (B) Quantitation of panel A. Western blot analysis demonstrated reduced binding of EBP1 p48 to DPPA4, normalized to p48 input, in differentiated NT2s. (n=3, *p<0.05). Error bars are standard error of the mean (S.E.M). (C) Downregulation of OCT4, DPPA4 and EBP1 protein expression by western blot analysis to verify differentiation of NT2s with at-RA.
