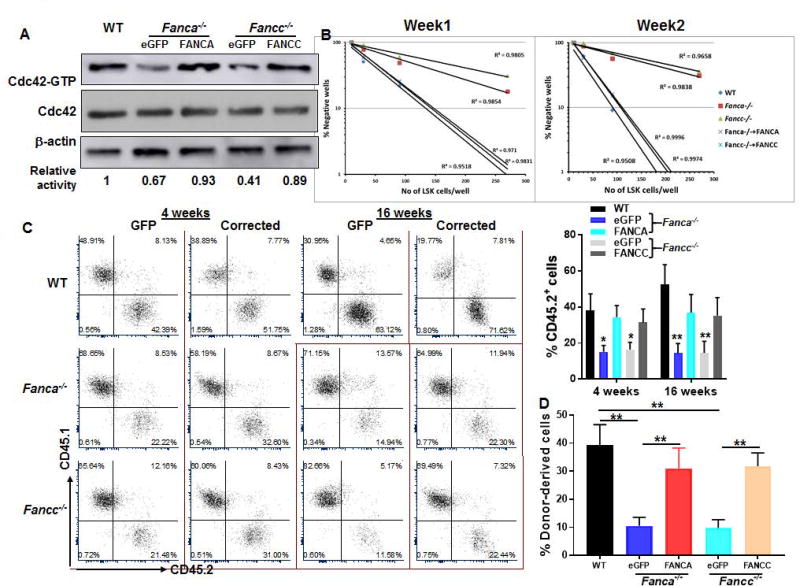
Fig. 3. Genetic correction of FA deficiency restores Cdc42 activity and supportive function of FA MSC.
(A) Genetic correction of FA deficiency restores Cdc42 activity in FA MSCs. MSCs isolated from WT, Fanca−/− or Fancc−/− mice were transduced with retroviral vector expressing eGFP, eGFP-FANCA, or eGFP-FANCC. Sorted GFP+ cells were subjected to in vitro Cdc42 pull down assay. The level of active, GTP-bound Cdc42, total Cdc42 and β-actin were determined. (B) Complementation of FANCA or FANCC improves CFAC of FA MSCs. Sorted GFP+ MSC cells described in (A) were co-cultured with graded numbers of flow sorted WT LSKs. Cultures were maintained in 40% methyl cellulose medium for two weeks and the colonies were counted on week 1 and 2. Group of at least 6 phase dim cells were counted as one colony. (C) Genetic correction of FA deficiency improves reconstitution of co-cultured WT HSCs in irradiated recipients. Co-cultured WT LSK cells described in (B) were transplanted into lethally irradiated BoyJ recipients. Donor-derived chimera was detected by flow cytometry analysis at 4 and 16 weeks post-transplant. (D) Complementation of FA deficiency improves long-term repopulating capacity of co-culture cells. WBMCs from the primary recipients were transplanted into sublethally irradiated BoyJ recipients. Donor-derived chimera were determined 16 week post transplant. Representative flow plots and quantifications were shown. Results are means ± standard deviation (SD) of 3 independent experiments (n=9 per group). *p<0.05; ** p<0.01.