Table 2.
Hydrogenation of different diene and triene substrates using C8 PdNPa
| Substrate | 1,2-Add | Yield (%) | 1,4-Add | Yield (%) | 1,4-Add/1,2-add |
|---|---|---|---|---|---|
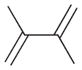 1 |
 1a |
9 |
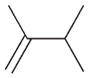
 1b |
91 | 10.4 |
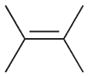
 2 |
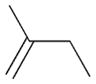 2a |
7 |
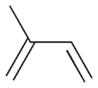
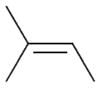 2c |
93 | 14.2 |
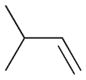 2b |
0 | ||||
|
3b |
3a |
92c E/Z = 4.0 |
3a |
— | — |
|
3b |
8 | ||||
|
4 |
4a |
29 |
4c |
71 E/Z = 3.5 | 2.4 |
|
4b |
0 | ||||
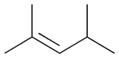
|
5b |
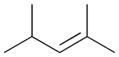 5a |
90c |
 5a |
— | — |
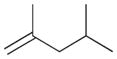 5b |
7 | ||||
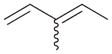 6b |
 6a |
90c |
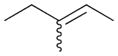
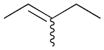 6a |
— | — |
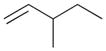 6b |
5 | ||||
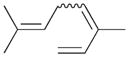 7 |
 7a |
41 |
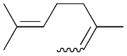 7c |
59 | 1.5 |
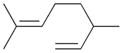 7b |
0 | ||||
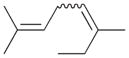
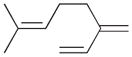 8 |
 8a |
3 |
 7c |
69 | 3.0 |
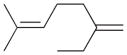 8b |
20 |
Reaction conditions: 0.5 mmol diene, 5 mol% Pd catalyst, 2.5 mL CDCl3, 1 atm H2, 24 h reaction. Yields were obtained by 400 MHz 1H NMR.
The catalytic reaction of this substrate yields the same product after 1,2- and 1,4-addition reactions.
This yield is the combination of both 1,2- and 1,4-addition reactions.
