Figure 5.
HuSC Phenotype and Function after Cryopreservation
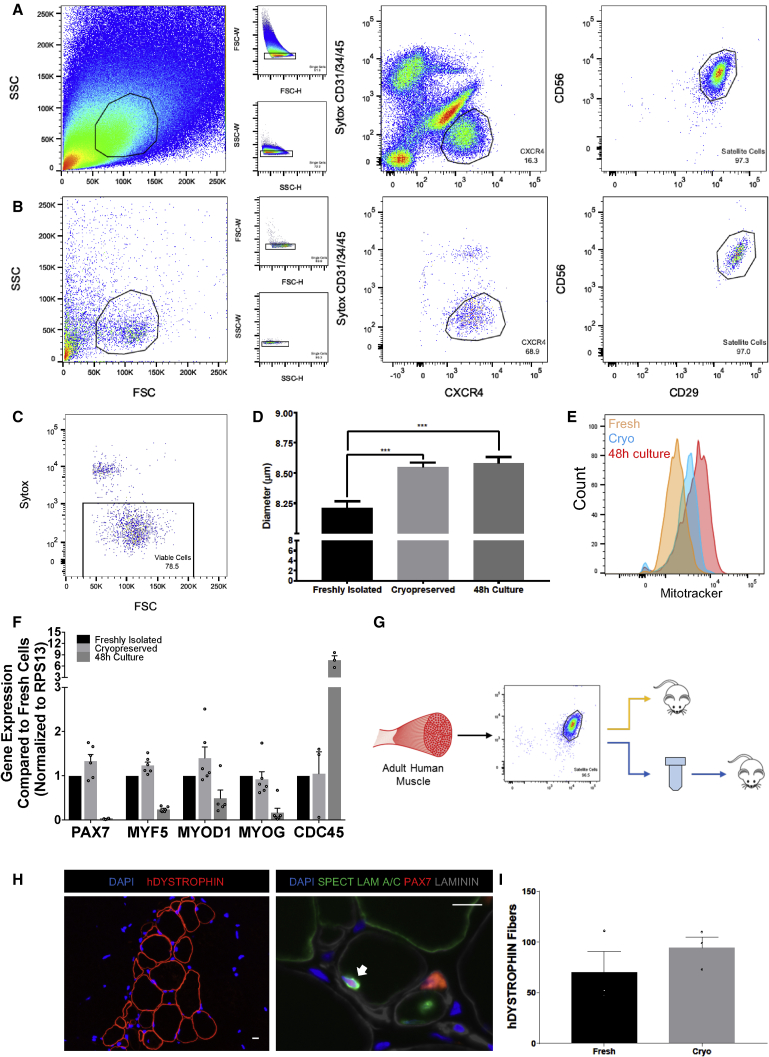
(A) Flow-cytometry profile of an HuSC isolation from the rectus abdominis muscle of a 43-year-old male.
(B) Isolated HuSCs (A) were cryopreserved and thawed, then restained and sorted. In (A and B), cells gated are outlined in black within each plot. The percentage of events in each gating step is shown in each plot.
(C) Sytox blue flow-cytometry profile for sorted satellite cells in (B) demonstrating >75% viability after cryopreservation.
(D) Representative bar graph showing the average difference in satellite cell size among freshly isolated, cryopreserved, and 48-hr cultured cells (n = 490, 2,037, and 1,942 cells, respectively, from three biological replicates). ∗∗∗p < 0.001.
(E) Representative histograms of freshly isolated satellite cells (orange), cryopreserved cells (blue), and 48-hr cultured cells (red) assessed by MitoTracker flow-cytometry assay (n = 3 biological replicates). “Count” denotes number of cells. Complete profiles are shown in Figure S5.
(F) Bar plots of qRT-PCR data comparing the expression of PAX7, MYF5, MYOD1, MYOG (n = 6), and CDC45 in satellite cells from freshly isolated, cryopreserved, and 48-hr cultured cell groups (n = 3). n Values denote technical replicates from two independent biological samples. Gene expression was normalized to the housekeeping gene RPS13.
(G) Schematic depicting experimental approach to compare the engraftment of cryopreserved and fresh HuSCs from the same muscle tissue.
(H) Representative images after transplantation with 2,000 cryopreserved HuSCs, of human fiber engraftment (left) and of repopulation the niche (right) with HuSCs (arrows) (n = 3 biological replicates). Scale bars, 10 μm.
(I) Bar graph depicting the engraftment of human fibers after transplantation with freshly isolated versus cryopreserved HuSCs quantified by human-specific DYSTROPHIN staining. There was no significant difference in engraftment (n = 3 individual mice).
Data presented as mean ± SEM. See also Figure S5.