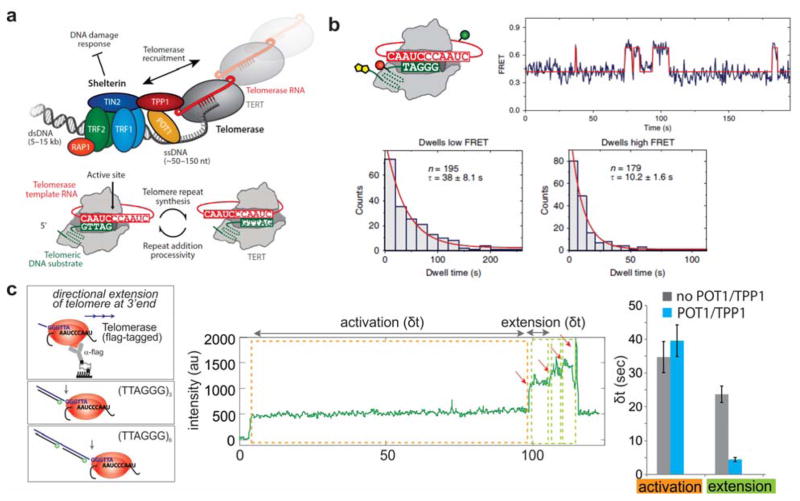
Figure 11. SM studies of the telomerase RNP holoenzyme.
(a) Top: Telomeres are protected from DNA damage by the Shelterin protein complex, and are extended by the RNA-templated telomerase reverse transcriptase. Bottom: The same six-nucleotide template portion of telomerase RNA is re-used to synthesize multiple telomeric repeats. Adapted with permission from Ref 238 Copyright 2017 by Annual Reviews. (b) After synthesis of a repeat, the DNA substrate fluctuates between different alignment registers with TR. The newly-formed RNA/DNA hybrid is eventually trapped in the active site for further extension. When the fluorophores are placed on TR and the DNA substrate, this results in fluctuations between low- and high-FRET states. Reproduced with permission from Ref 146 Copyright 2014 Macmillan Publishers. (c) Telomere extension detected by binding of fluorescent probes to telomeric repeats. After addition of dNTPs, an activation period is followed by an extension period in which multiple repeats are rapidly added. Right: the protein co-factors POT1 and TPP1 decrease the time required for extension without impacting the time required for activation. POT1 and TPP1 were also found to enhance repeat addition processivity. Reproduced with permission from Ref 239 Copyright 2014 Macmillan Publishers Limited.