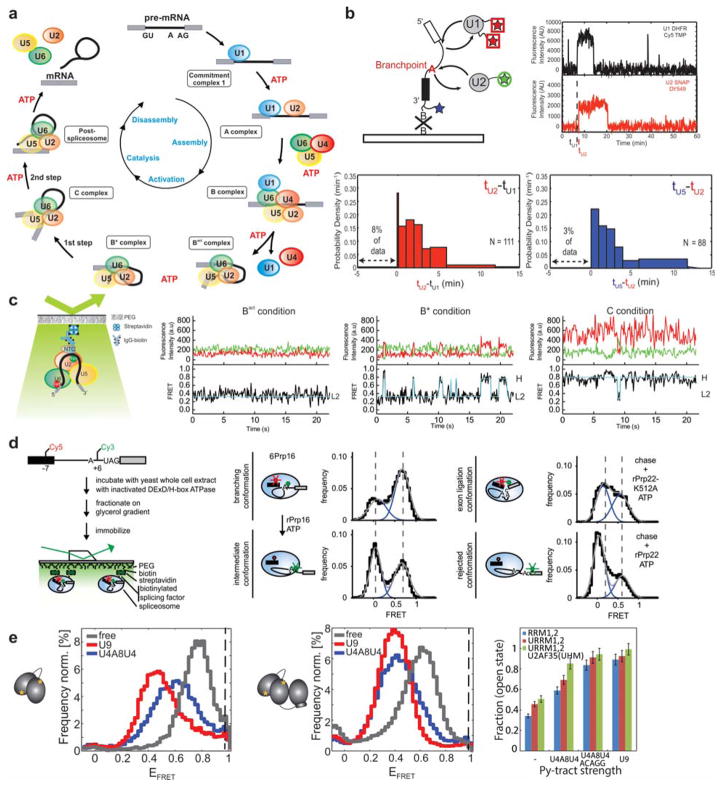
Figure 12. Assembly, catalytic activation, and regulation of the spliceosome.
(a) The splicing cycle, indicating binding and dissociation of the snRNPs and steps in which ATP hydrolysis is required to promote conformational rearrangements. (b) Spliceosome assembly was studied by monitoring the binding and dissociation of fluorophore-labeled snRNPs. Binding is reversible (upper right), but has a largely enforced order of U1, followed by U2, U5 (likely as part of the tri-snRNP), and the NTC (bottom). Reproduced with permission from Ref 149 Copyright 2011, American Association for the Advancement of Science (c) Study of the Bact-to-B*-to-C complex transition by single-molecule pull-down FRET with the BP and 5′SS labeled. FRET traces show the static mid-FRET state that characterizes the Bact complex, fluctuations between mid-FRET and high-FRET states that characterize the branching-competent B* complex, and relatively stable high-FRET state that characterizes the post-branching C complex. Reproduced with permission from Ref 143 Copyright 2013 Nature America, Inc. (d) Left: pre-mRNA conformations in stalled spliceosomes were studied following purification via glycerol gradient centrifugation. Middle: the helicase Prp16 can separate the branchpoint and 5′SS after branching. When the BP and 5′SS are labeled, this results in a shift of population to lower EFRET upon addition of Prp16. Right: the helicase Prp22 can separate the 5′SS and 3′SS before exon ligation. When the 5′SS and 3′SS are labeled, this results in a shift of population to lower EFRET upon addition of Prp22. Both of these rearrangements represent proofreading mechanisms by which suboptimal substrates can be rejected. Reproduced with permission from Ref 244 Copyright 2016 Elsevier Inc. (e) Left: U2AF65 exists largely in a high-FRET “closed” conformation in the absence of RNA (gray), and transitions to a mid-FRET “open” state upon binding to RNAs with strong Py-tracts (red). Weak Py-tracts cause an intermediate shift (blue). Middle: Addition of U2AF35 enables U2AF65 to adopt the open state even in the presence of weak Py-tracts. Reproduced with permission from Ref 245.