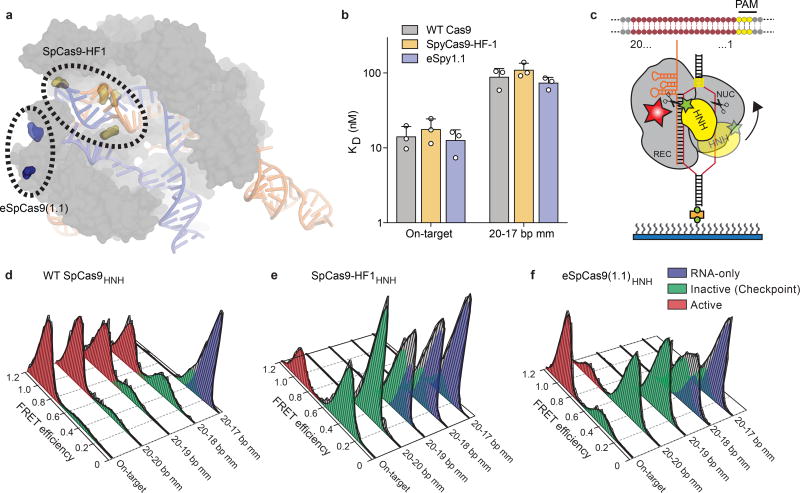
Figure 1. High-fidelity Cas9 variants enhance cleavage specificity through HNH conformational control.
a, Locations of amino acid alterations in existing high-fidelity SpCas9 variants mapped onto the dsDNA-bound SpCas9 crystal structure (PDB ID: 5F9R); HNH domain is omitted for clarity. b, Dissociation constants with mean and s.d. shown; n = 3 independent experiments (overlaid as white circles). c, Cartoon of DNA-immobilized SpCas9 for measuring HNH conformation by smFRET, with DNA target numbering scheme. d–f, smFRET histograms showing HNH conformation with indicated Cas9 variants bound to on-target and mismatched targets using nucleotide numbers diagramed in panel c. Black curves represent a fit to multiple Gaussian peaks.