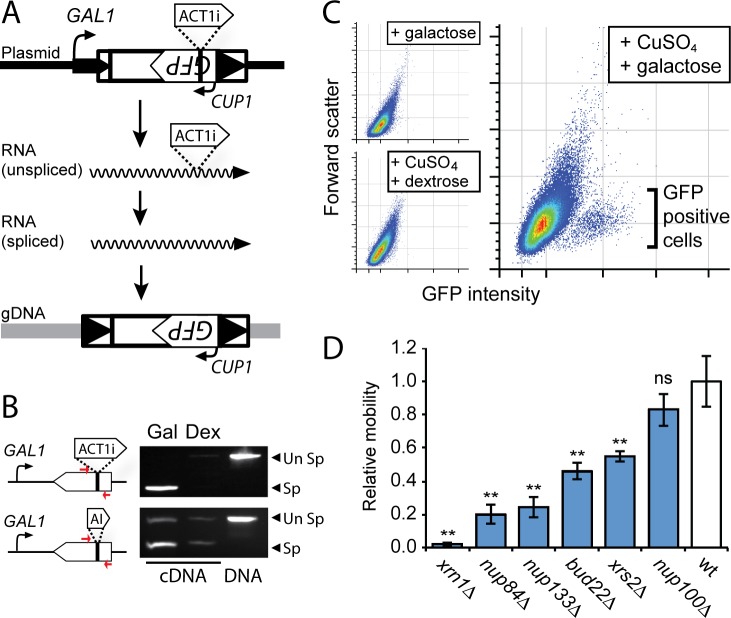
Fig 3. A novel GFP-based reporter of Ty1 mobility.
(A) An overview of the GFP-tagged Ty1 plasmid. Ty1 transcription is induced by activation of the GAL1 promoter that produces a long Ty1 transcript including an internal GFP gene and an ACT1 intron (ACT1i). The spliced transcript has the ACT1i removed, which then provides a template for Ty1 protein production and reverse transcription. Ty1 cDNA is imported into the nucleus and integrated into the S. cerevisiae genome. The GFP gene is then induced from the CUP1 promoter by CuSO4 to report successful integration events. (B) RT-PCR was used to assess splicing of RNA with ACT1i versus an artificial intron (AI) within the GFP gene (primer positions marked by red arrows). Spliced RNA transcripts (Sp) were mainly detected upon induction of the transcription by the GAL1 promoter using galactose (Gal). Growth on dextrose (Dex) inhibits the GAL1 promoter and the production of RNA transcripts. Plasmid DNA was used as a positive control to allow the PCR amplification across intron-containing GFP. “Un Sp” indicated the detection of unspliced RNAs. (C) Flow cytometry analysis shows that GFP is only expressed under conditions of galactose induction of Ty1 expression followed by CuSO4 induction of GFP. (D) The effect of six different gene deletions on Ty1 mobility, relative to wild-type S. cerevisiae. The relative mobility was measured as a percent of GFP positive cells after induction of the Ty1-GFP reporter, and was repeated independently, three times (error bars: standard error, n>3; **Tukey–Kramer method, p<0.05). All values are normalized to wildtype.