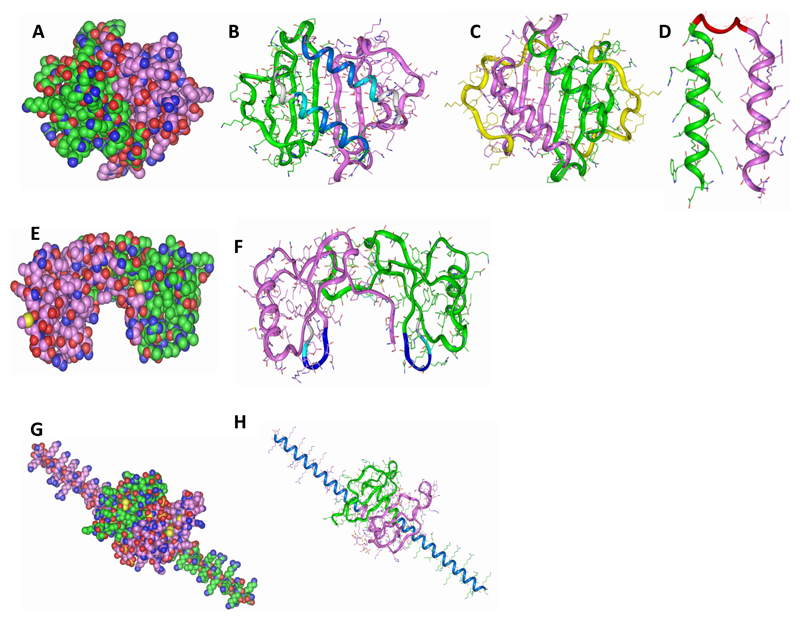
Figure 1. Modelling peptides based on chemokines.
(A) Wildtype CXCL8 (B) C-terminal peptides (pCXCL8-1 and -2) indicated in blue where -1 is dark blue and -2 is the longer peptide indicated by two blue shades, (C) longer peptide (pCXCL8-3) including all known HS binding sites as shown by the green and purple structures and the yellow highlighting the N-terminal residues which were removed, (D) both C-terminal alpha helices (pCXCL8-4) linked together by a pre-modelled linker to form a “dimer”. (E) Wildtype CCL5. (F) Indicated are peptides based on the 40s loop of CCL5 (pCCL5-1/-2/-3) with pCCL5-3 being the longest indicated by two blue shades and grey, pCCL5-2 two blue shades and pCCL5-1 dark blue. (G) Wildtype CXCL12γ. (H) C-terminal peptide (pCXCL12-1) indicated in blue. Carbon atoms are seen in green and pink where each represents the monomeric unit, oxygen in red, nitrogen in blue and sulphur in yellow (A, E and G). Structures are shown as dimers. Please note that CXCL8 and CXCL12 peptides do not form the helical structures as depicted but are based on the helical sequences present in the wildtype chemokine.