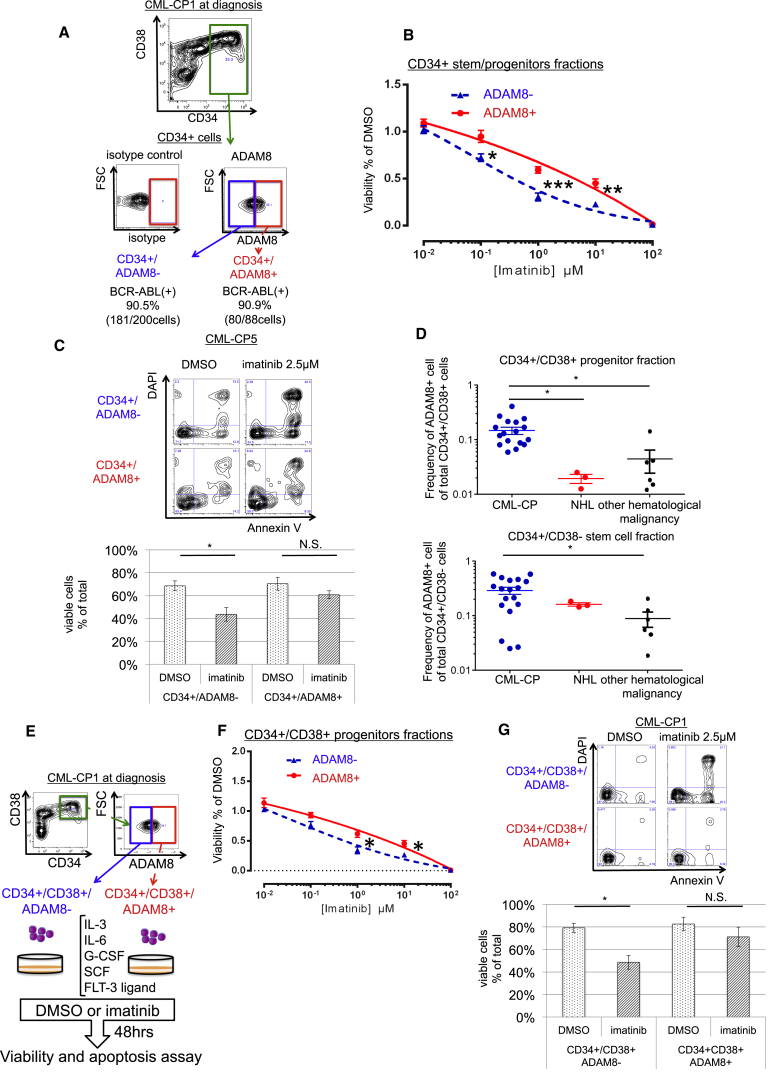
Figure 4.
ADAM8 Marks TKI-Resistant CML Cells in BM Samples of Patients with Newly Diagnosed CML-CP
(A) A scheme of viability and apoptosis assay for ADAM8+ cells in CD34 + stem/progenitor fraction.
(B) Viability assay for CD34+/ADAM8+ cells in BM of newly diagnosed CML-CP patients. Data show means ± SEM, n = 6 patients (p values: two-tailed Student's t test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
(C) Apoptosis assay for CD34+/ADAM8+ cells in BM of newly diagnosed CML-CP patients. Representative FCM data are shown on the left; 2.5 μM imatinib for 48 hr, which significantly induces apoptosis in ADAM8− cells, does not induces apoptosis in ADAM8+ cells. Data show means ± SEM, n = 4 patients (p values: two-tailed Student's t test; ∗p < 0.05).
(D) Frequency of ADAM8+ cells in CD34+/CD38+ progenitor fraction and CD34+/CD38−stem cell fraction. In the progenitor fraction, the frequency of ADAM8+ cells is significantly higher in CML-CP patients (n = 18) than in non-Hodgkin lymphoma (n = 3) and other hematological malignancies (AML, n = 1; acute lymphocytic leukemia, n = 1; myelodysplastic syndrome, n = 1; chronic myelomonocytic leukemia, n = 3). Data show means ± SEM, n = shown above patients (p values: two-tailed Student's t test; ∗p < 0.05).
(E) A scheme of viability and apoptosis assay for ADAM8+ cells in CD34+/CD38+ stem/progenitor fraction.
(F) Viability assay for CD34+/CD38+/ADAM8+ cells in BM of newly diagnosed CML-CP patients. Data show means ± SEM, n = 4 patients (p values: two-tailed Student's t test; ∗p < 0.05).
(G) Apoptosis assay for CD34+/CD38+/ADAM8+ cells in BM of newly diagnosed CML-CP patients. Representative FCM data are shown on the left; 2.5 μM imatinib for 48 hr, which significantly induces apoptosis in ADAM8− cells, does not induce apoptosis in ADAM8+ cells. Data show means ± SEM, n = 6 patients (p values: two-tailed Student's t test; ∗p < 0.05).