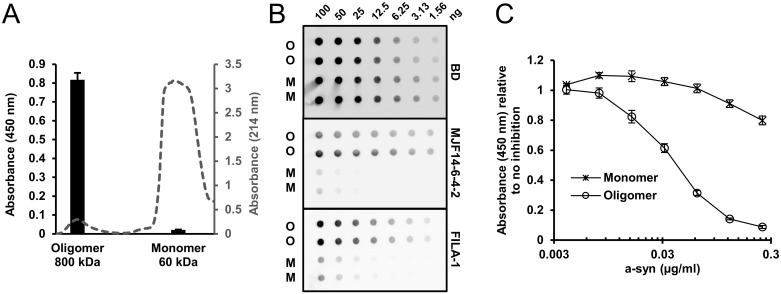
Fig 1. Specificity and affinity of the MJF14-6-4-2 antibody.
A. Monomers and oligomers were isolated by gel filtration and eluted in two distinct fractions with peak values corresponding to 60 kDa and 800 kDa, respectively (grey broken line). The absorbance profile at 214 nm is indicated at the right ordinate axis. To validate the specificity of the oligomer ELISA we incubated antibody coated wells with the same concentration of monomer and oligomer (9 ng/mL) and used a monoclonal anti-a-syn antibody as second antibody. Bound antibody was detected by anti-mouse HRP and the TMB color change was measured as absorbance at 450 nm. The ELISA gives a strong signal for oligomers whereas there is only a negligible signal from the monomer sample (black bars). Background absorbance was subtracted. The ELISA signal (absorbance at 450 nm) is presented at left ordinate axis. B. We compared the aggregate specificity of the MJF14-6-4-2 antibody with the well characterized polyclonal antibody FILA-1 using dot blotting analysis. Dilutions of monomers (M) and oligomers (O) were immobilized and tested for their antibody binding. The non-conformation dependent monoclonal a-syn antibody (BD) (0.5 μg/ml) demonstrated equal loading of monomer and oligomer. MJF14-6-4-2 (Abcam) (2.2 ng/ml) and FILA-1 (3.5 μg/ml) antibodies display the same extend of selectivity for the aggregated a-syn. C. The affinity of MJF14-6-4-2 antibody was assessed by a direct ELISA on oligomer coated wells. The MJF14-6-4-2 (15.6 ng/ml) antibody was pre-incubated overnight with increasing concentrations of monomer or oligomer before being applied to the oligomer containing wells. The concentration of oligomer that gave 50% inhibition was measured to be 42 ng/ml. The KD-value of MJF14-6-4-2 was calculated to be 2.9 · 10−10 M based on the assumption that 10 a-syn monomers are required to generate a folding specific MJF14-6-4-2 epitope. Both A and C show one representative experiment of 3. Standard deviations represent three technical replicates.