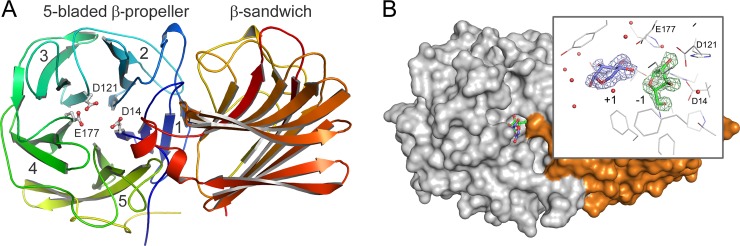
Fig 1. Overall fold of Xyl.
A. Ribbon representation of Xyl in rainbow colors from blue (N-terminus) to red (C-terminus). The blades of the N-terminal five-bladed β-propeller catalytic domain are numbered 1–5. The catalytic residues are shown as sticks and colored by element (carbon, gray; oxygen, red). B. Surface representation showing the active site of Xyl with bound β-L-arabinofuranose (carbon atoms in green) and α-D-xylopyranose (carbon atoms in blue) in the Xyl•arabinose•xylose complex structure (PDB 5Z5I). The N- and C-terminal domains are colored in gray and orange, respectively. Inset: The bound monosaccharides in the active site are shown with the Fobs—Fcalc electron density map, obtained prior to refinement, and contoured at 0.5 σ. The subsites of the Xyl’s active site are indicated as -1 and +1. If not specified otherwise, all figures were prepared using the program PyMOL [The PyMOL Molecular Graphics System, v. 0.99, Schroödinger, LLC, http://www.pymol.org].