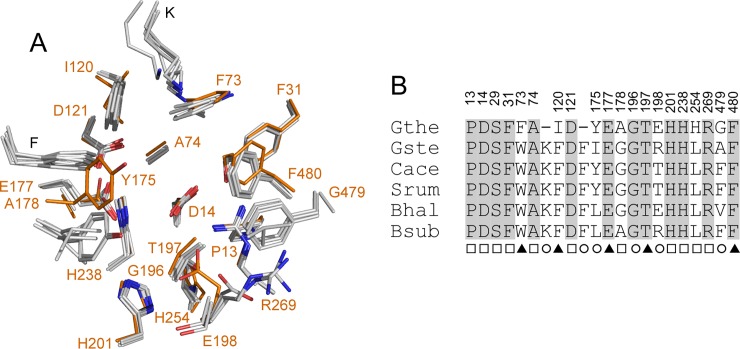
Fig 3. Active site residues in GH43 β-1,4-xylosidases.
A. Superposition of the active sites of the β-1,4-xylosidases from G. stearothermophilus (Gste; PDB 2EXH), C. acetobutylicum ATCC 824 (Cace; PDB 1YI7), B. halodurans C-125 (Bhal; PDB 1YRZ), S. ruminantium GA192 (Srum; PDB 3C2U), and B. subtilis subsp. subtilis str. 168 (Bsub; PDB 1YIF) onto that of Xyl (Gthe; carbon atoms in orange). Residues are numbered according to Xyl. B. The superposition in A is presented as a sequence alignment and the strictly conserved residues are grey-shaded. The numbering above the alignment refers to Xyl. The marks below the residues indicate their position 5 Å around the subsite -1 (□), +1 (○), or both (▲) of the two-subsite active site of GH43 β-1,4-xylosidases. Fig 3B was prepared manually.