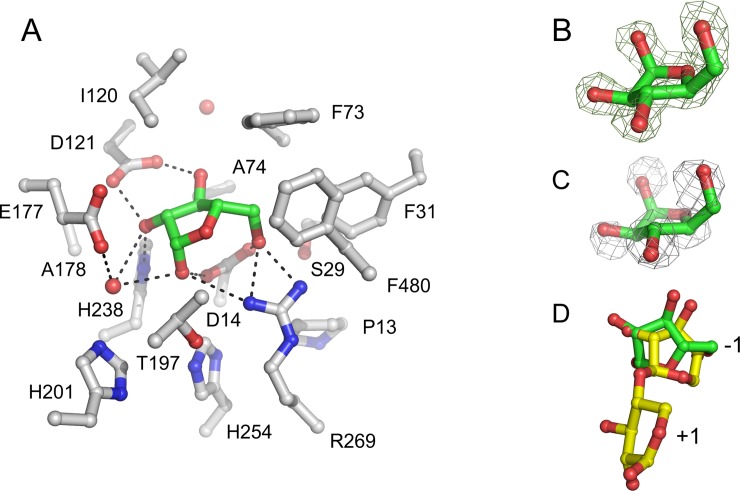
Fig 4. Subsite -1 structure of the active site of Xyl.
A. The interactions of β-L-arabinofuranose (carbon atoms in green) with Xyl in subsite -1 of the Xyl•arabinose structure. The amino acid side chains (carbon atoms in gray) and two water molecules (red spheres) within 5.0 Å from the monosaccharide are shown. The protein orientation is approximately the same as in Fig 1. Potential hydrogen bonds between the monosaccharide and active site residues and a water molecule are represented as dashed lines. B. Fobs—Fcalc electron density of bound β-L-arabinofuranose, obtained prior to refinement, contoured at 0.5 σ. C. A superposition of the Xyl•arabinose and Xyl•xylose structures revealed that all arabinose oxygen atoms are at similar positions as water molecules in the Xyl•xylose structure. The 2Fobs—Fcalc electron density map contoured at 1 σ is shown for the five water molecules in subsite -1 of the Xyl•xylose structure. D. A superposition of the active site residues of Xyl and G. stearothermophilus β-1,4-xylosidase (its active site subsites are indicated as -1 and +1) [17], showing that arabinose (carbon atoms in green) bound in subsite -1 of Xyl is shifted by ~1 Å relative to the xylosyl moiety in that of G. stearothermophilus β-1,4-xylosidase (yellow).