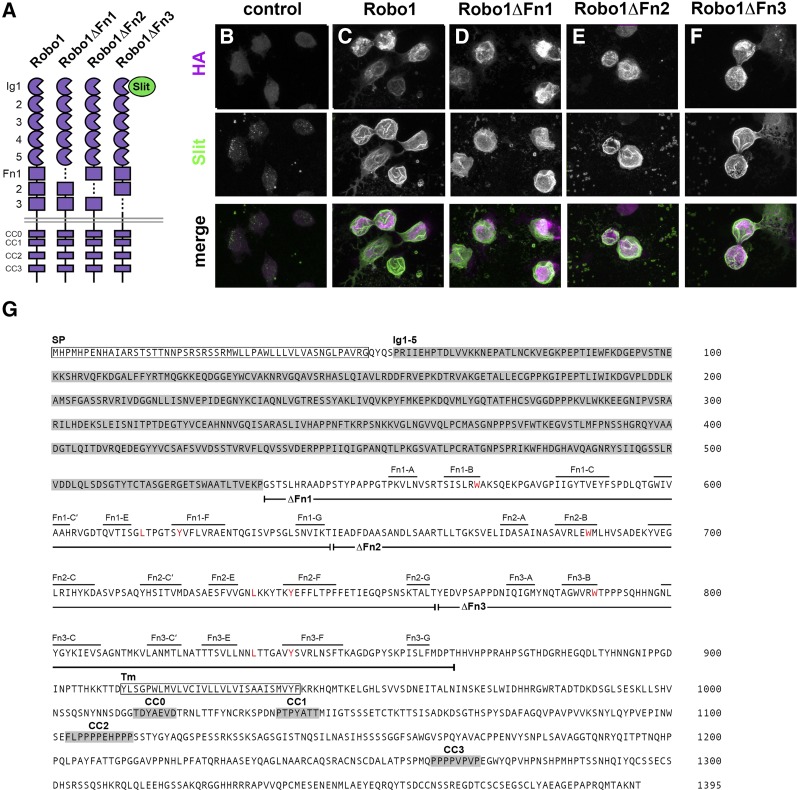
Figure 1.
Deletion of individual Fn domains does not interfere with Robo1’s ability to bind Slit in cultured Drosophila S2R+ cells. (A) Schematic of the tested Robo1 variants. (B–F) Cells were transfected with HA-tagged Robo1 variants and treated with Slit-conditioned media. After Slit treatment, cells were stained with anti-Slit antibody to detect bound Slit (green) and anti-HA antibody to detect HA-tagged Robo1 variants (magenta). Slit binds only weakly to mock-transfected cells (B), but binds robustly to cells expressing full-length Robo1 (C) or any of the three Fn deletion variants (D–F). (G) Robo1 protein sequence highlighting conserved structural features and illustrating the extent of individual Fn domain deletions. Fn domains have been reannotated based on revised predictions of β-strand locations (annotated above the protein sequence). β-strand nomenclature follows that of Campbell (1994) and Leahy (1996). Amino acids highlighted in red represent a conserved tryptophan residue in strand B, a conserved leucine residue in the E–F loop, and a conserved tyrosine residue in strand F (Leahy 1996). CC, conserved cytoplasmic motif; Fn, fibronectin type-III repeat; Ig1–5, immunoglobulin-like domains 1–5; SP, signal peptide; Tm, transmembrane helix;.