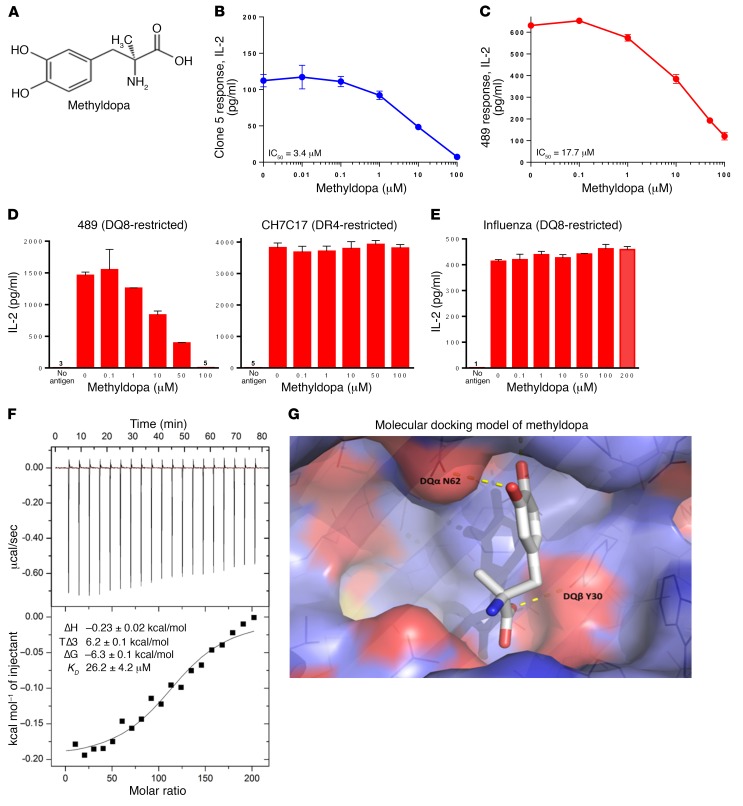
Figure 3. Methyldopa blocks DQ8 antigen presentation in vitro.
(A) Methyldopa chemical structure. Methyldopa blocked an in vitro DQ8–restricted T cell response by (B) clone 5 to insulin B:13-23 and (C) TCR 489 to deamidated α-gliadin presented by DQ8. The negative controls (no antigen added to culture) resulted in IL-2 levels below 3 pg/ml. (D) Methyldopa specifically inhibited 489, a DQ8-restricted T cell response, while not altering C7CH17, a DR4-restricted T cell response to the HA peptide HA306-318. Cells from an EBV-transformed B cell line homozygous for DQ8 and DR4 were used as APCs in this experiment, such that each APC had DQ8 and DR4 on the cell surface. (E) Methyldopa did not inhibit a DQ8-restricted T cell response to the influenza peptide HA102-118. (B–E) Data represent the mean ± SEM and are representative of 3 independent experiments. (F) Representative isothermal titration calorimetric data for titration of methyldopa into DQ8 protein. Top: Heat released as a function of time from 2-μl injections of 4 mM methyldopa titrated into the sample cell containing 4 μM DQ8 protein at 25oC. Bottom: Fitted binding curve along with the measured thermodynamic parameters and the calculated KD from 3 independent experiments, reported as the mean ± SEM. (G) Molecular docking model of methyldopa in the DQ8 antigen–binding cleft shows potential H bonds between a methyldopa hydroxyl group and DQα62 asparagine (DQα N62) on the DQα-helix and between the methyldopa carboxylic acid and DQβ30 tyrosine (DQβ Y30) on the floor of the cleft.