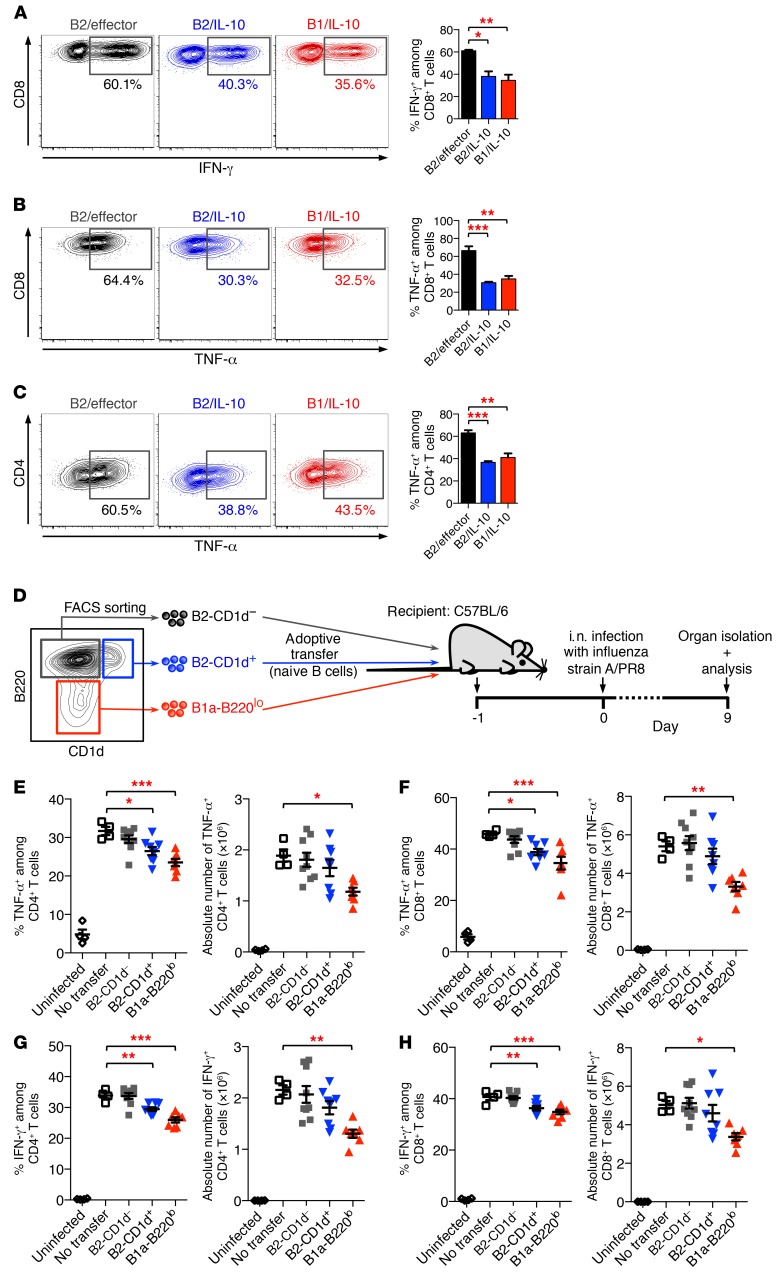
Figure 5. Suppressive function of IL-10–producing Bregs.
(A–C) Bregs suppress inflammatory cytokine production in T cells in vitro. B cells from IL-10/GFP reporter (Vert-X) mice were treated for 16 hours with LPS, and PMA/ionomycin was added during the last 5 hours. B2/effector cells (B220hiGFP–), B2/IL-10 cells (B220hiGFP+), and B1/IL-10 cells (B220loGFP+) were then purified by FACS and were subsequently cocultured with naive CD8+ T cells (A and B) or naive CD4+ T cells (C). Cultures were stimulated with anti-CD3 for 48 hours and restimulated with PMA/ionomycin in the presence of BFA/monensin for 5 hours. Percentage of IFN-γ–producing (A) and TNF-α–producing (B and C) T cells was determined by intracellular cytokine staining. (D–H) Bregs suppress inflammatory cytokine production in T cells during antiviral immune response in vivo. (D) Experimental model. Briefly, naive CD19+B220hiCD1d– B2 B cells (gray), CD19+B220hiCD1d+ B2 B cells (blue), and CD19+B220lo B1a cells (red) were purified from Toso–/– (KO) mice by FACS and were adoptively transferred into C57BL/6J mice. Mice were infected i.n. with 1,000 PFU influenza virus strain A/PR8 (H1N1). On day 9 p.i., lung cells were isolated and analyzed for cytokine staining. (E–H) Number and frequency of TNF-α–producing (E and F) and IFN-γ–producing (G and H) CD4+ T cells (E and G) and CD8+ T cells (F and H). Mice that had not received adoptively transferred cells (no transfer; open squares) but were also infected with influenza were used as positive control; uninfected mice served as a negative control (open circles). Data are expressed as mean ± SEM; symbols represent individual mice. (A–C) n = 3; (E–H) n = 4–9. *P < 0.05; **P < 0.01; ***P < 0.001; 1-way ANOVA and Dunnett’s post hoc test. Data are representative of at least 3 independent experiments.