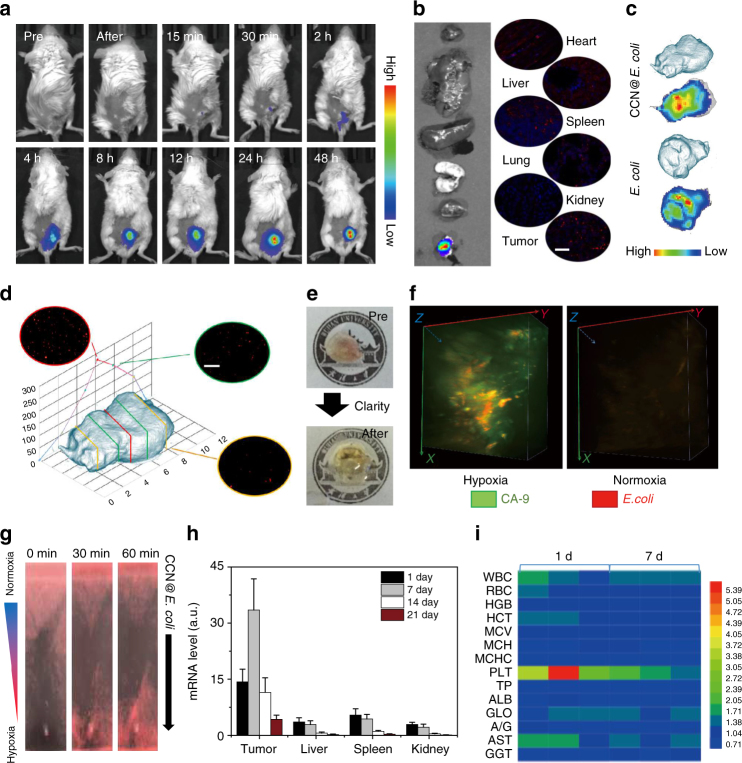
Fig. 3.
In vivo bio-distribution and bio-compatibility of PMT system. a In vivo fluorescence imaging of tumor-targeting ability of CCN@E. coli. The imaging was performed at different time points after the i.v. injection of CCN@E. coli (108 CFU mL−1 in 200 μL saline, n = 5). b Ex vivo fluorescence imaging for investigating the bio-distribution of CCN@E. coli (Scale bar: 100 μm). c Tumor accumulation of E. coli and CCN@E. coli. d Transverse sections of CCN@E. coli treated tumor after targeting and penetrating (Scale bar: 100 μm). The experiment was performed at 24 h after the i.v. injection of CCN@E. coli (108 CFU mL−1 in 200 μL saline, n = 3). e Images of whole mouse tumor and CLARITY technique treated optically transparent tumor. The experiment was performed at 24 h after the i.v. injection of CCN@E. coli (108 CFU mL−1 in 200 μL saline). f Three-dimensional fluorescence imaging for visualizing the co-localization of CCN@E. coli and hypoxic region within the tumor. g In vitro hypoxia-induced chemotaxis of CCN@E. coli. h PCR analysis of CCN@E. coli within liver, spleen, and kidney decreased with the time prolonging. The PCR analysis was performed on the 1st, 7th, 14th, and 21th days after the i.v. injection of CCN@E. coli (108 CFU mL in 200 μL saline, n = 10). The mean values and S.D. are presented. i Blood biochemistry and hematologic indexes of mice after i.v. injection with CCN@E. coli. The experiment was performed on the 1st and 7th days after the i.v. injection of CCN@E. coli (108 CFU mL−1 in 200 μL saline, n = 3)