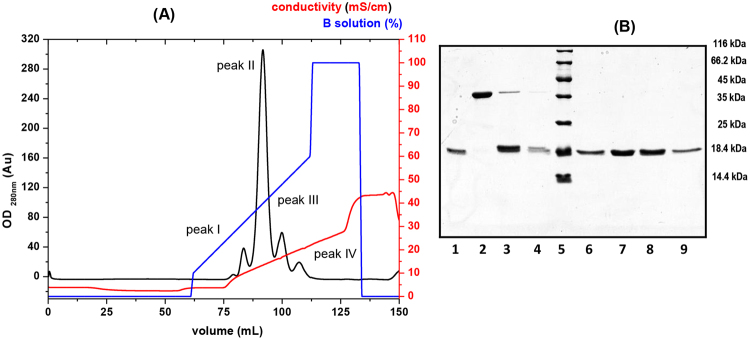
Figure 2.
Purification of StIW111C homodimers stabilized by disulfide bridge. (A) Profile of ion-exchange chromatography of the StI W111C mutant on a Sepharose-SP Fast Flow column (r/L: 0.5/7.0 cm). The procedure was performed at 76.4 cm/h lineal flow and 1 mL fractions were collected on ÄKTA primer chromatography system. Purification was carried out from protein eluted from carboxymethyl cellulose (CM-52) column (Fig. S2). A280 nm (OD280 nm) (black line), gradient of NaCl concentration in solution B (blue line) and conductivity (red line) are shown. (B) SDS-PAGE 15% of acrylamides83. Lanes 1, 2, 3 and 4: eluting fractions from peak I, II, III and IV without incubated with 2-mercaptoethanol (2-ME), respectively. Lanes 6, 7, 8 and 9: eluting fractions from peak I, II, III and IV incubated with 2-ME, respectively. Lane 5: Pierce unstained protein molecular weight marker (Thermo Scientific; USA).