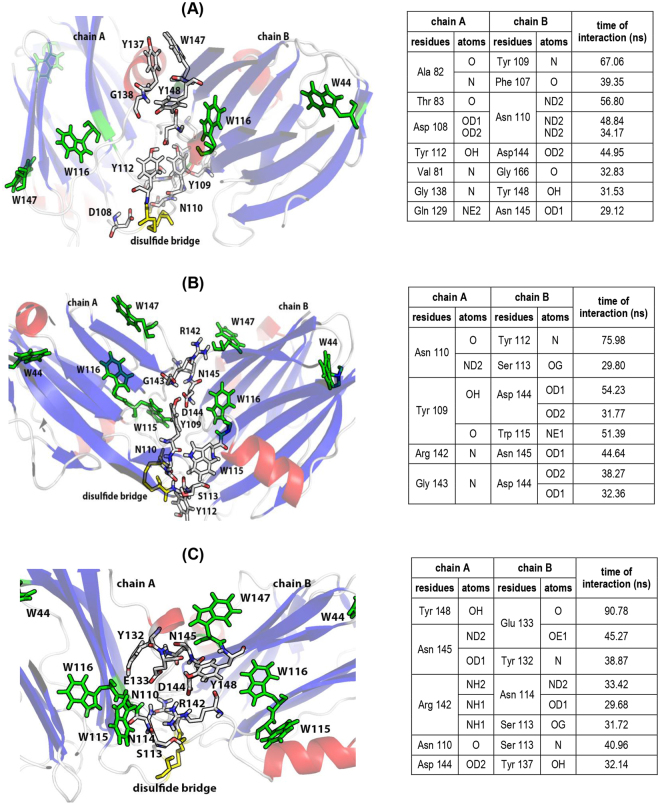
Figure 8.
Inter-protomer interfaces of three StI W111C homodimer structural models stabilized by a disulfide bridge between Cys 111 during molecular dynamics. All homodimer models were achieved after 100 ns of the trajectory (A) model_14 (31a_32b_1bsr), (B) model_25 (32a_31b11ba), and (C) model_63 (90a_90b_1grg). The different elements of secondary structure are shown in red (helixes), blue (sheets) and gray (loops and unordered structures). Tryptophan residues (green), except W147 in model_14, and disulfide bridge (yellow) are also high-lighted. Tryptophan and amino acid residues involved in the protein-protein interaction are labeling. Tables inserted (right) summarize the hydrogen bounds established for more than 30 ns of the dynamics (>30% of the simulation time). Images were produced with the Pymol v1.7.6.6 software for Windows107.