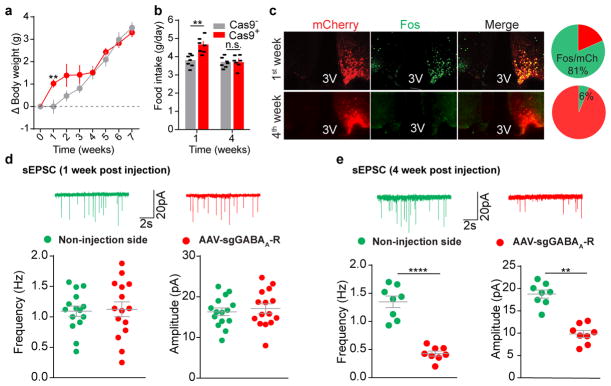
Extended Data Figure 8. Dynamic changes of neuronal activity and synaptic neurotransmission following CRISPR-mediated deletion of GABAA receptors in AgRP neurons.
a, b, Body weight measurement (a) and daily food intake (b) of Agrp-IRES-Cre (Cas9−) and Agrp-IRES-Cre::LSL-Cas9-GFP (Cas9+) mice following bilateral injection of AAV-sgGABAA-R into the ARC (n=7 mice per group). c–f, Representative sections and quantification of mCherry::Fos co-immunostaining (c, n=3 mice per group), representative traces and quantification of spontaneous excitatory postsynaptic currents (sEPSCs) (d, n=15 neurons from 3 mice, e, n=8 neurons from 2 mice) in AgRP neurons of Agrp-IRES-Cre::LSL-Cas9-GFP mice after 1-week or 4-week unilateral injection of AAV-sgGABAA-R into the ARC. Reduced frequency and amplitude of sEPSCs were observed in AgRP neurons in a later stage following GABAA-R deletion, together with concurrent elimination of the observed disinhibition of these neurons (c), body weight gain (a), and hyperphagia (b), suggesting that excitatory and inhibitory afferents dynamically cooperate to modulate AgRP neuronal activities. In addition, the compensatory reduction in sEPSCs in virus-transduced neurons but not in the neurons from the contralateral side also indicate a cell-autonomous mechanism. Data are mean ± s.e.m. and representative of three independent experiments; **P<0.01, ****P<0.0001; Student’s two-tailed, unpaired t-test (b, d, e) or two-way ANOVA analysis with Šidák post hoc test (a).