Abstract
LIM and SH3 protein 1 (Lasp-1), a focal adhesion protein, serves a critical role in the regulation of cell proliferation and migration. The role of Lasp-1, as well as the intracellular signaling pathways involved in metastasis and progression of colorectal carcinoma, remains unclear. In the present study, the regulatory effect of Lasp-1 and the underlying molecular mechanism involved in migration and invasion of colorectal carcinoma were investigated. RNA interference and overexpression in SW480 cells were performed to elucidate the role of Lasp-1. Reverse transcription-quantitative polymerase chain reaction, western blotting and immunofluorescence were conducted to determine the mRNA and protein levels of Lasp-1 and extracellular-signal-regulated kinase 1/2 (ERK1/2) in SW480 cells as well as tumor and adjacent normal tissues obtained from 20 patients with colorectal carcinoma. Furthermore, a cell proliferation assay, flow cytometric analysis, and cell migration and invasion assays were performed to examine the effect of Lasp-1 on cell growth. The results of the present study demonstrated that Lasp-1 and ERK1/2 were upregulated in tumor tissue compared with adjacent normal colorectal mucosa. Cell-based in vitro assays demonstrated that Lasp-1 knockdown suppressed the expression and activation of ERK1/2, whereas Lasp-1 overexpression resulted in ERK1/2 upregulation. Additionally, Lasp-1 knockdown inhibited cell proliferation, migration, and invasion and induced cellular apoptosis and G0/G1 cell-cycle arrest. The results of the present study indicate that Lasp-1 serves a critical role in the progression of colorectal carcinoma regulating the activation of the mitogen-activated protein kinase signaling pathway.
Keywords: LIM and SH3 protein 1, mitogen-activated protein kinase, proliferation, metastasis, colorectal carcinoma
Introduction
Colorectal carcinoma is a common malignancy worldwide (1,2). It is the second leading cause of cancer-associated mortality and accounts for 10% of the cancer-associated mortalities worldwide (1,2), even though the screening techniques have improved markedly (3). Metastatic colorectal carcinoma is frequently an incurable disease (4,5). The underlying molecular mechanism involved in the progression of metastatic colorectal carcinoma remains to be elucidated.
LIM and SH3 protein 1 (Lasp-1) is a specific focal adhesion protein that serves an important role in the regulation of cell proliferation and migration (6,7). It has been reported to be upregulated in a number of malignant tumors, including clear cell renal (8), non-small cell lung (9), prostate (10), gallbladder (11), bladder (12,13), breast (14), hepatocellular (15,16) and colorectal (17,18) carcinomas. The role of Lasp-1 in metastasis and progression of colorectal carcinomas has been suggested previously (17). It has been demonstrated that Lasp-1 promotes proliferation and metastasis and induces cell cycle arrest at the G2/M phase by downregulating S100 calcium-binding protein P via the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway in gallbladder cancer (11). Lasp-1 overexpression has been positively associated with lymph node metastasis and poor prognosis in patients with cholangiocarcinoma (19). It has also been demonstrated that knockdown of Lasp-1 in HCCC-9810 human cholangiocarcinoma cells significantly increased cellular apoptosis and inhibited cell migration, invasion and proliferation (19). Furthermore, silencing of the Lasp-1 gene has been demonstrated to impair cell proliferation and migration of breast cancer cells (6). Lasp-1 overexpression in SW480 colorectal carcinoma cellshas been associated with an aggressive phenotype and poor prognosis in patients with clear cell renal (20) and colorectal (8) tumors. Additionally, depletion of Lasp-1 expression suppressed the transforming growth factor β (TGF-β) signaling pathway and inhibited epithelial-mesenchymal transition (20). Lasp-1 is localized to multiple sites of actin cytoskeleton assembly, including the focal adhesion, lamellipodia and filopodia (21). The expression and nuclear localization of Lasp-1 have been positively associated with malignancy, tumor grade and metastatic lymph node status (14). However, the intracellular signaling pathways involved in the metastasis and progression of colorectal carcinoma remain unclear. Lasp-1 induced the phosphorylation of proteins involved in themitogen-activated protein kinase (MAPK) signaling pathway (20). It has been demonstrated that microRNA (miR)-133α downregulated the expression of Lasp-1, by inhibiting the phosphorylation of extracellular-signal-regulated kinase (ERK)1/2 and MAPK/ERK kinase (MEK), and suppressed tumor growth and metastasis in liver and lung tumors (22). Further more, exogenous miR-133α inhibited the MAPK signaling pathway and induced the expression of epithelial markers (23). A similar effect on epithelial-mesenchymal transition has been observed following Lasp-1 targeting (22). The present study provides novel evidence for the role of Lasp-1 and the underlying molecular mechanism associated with the regulation of colorectal carcinoma progression.
Materials and methods
Patients
Colorectal cancer and adjacent normal tissue specimens were obtained from 20 patients (10 male and 10 female) (47±5.6 years old) between October 2015 and October 2016 who were treated in the Qianfoshan Hospital (Jinan, China). Written informed consent was obtained from all patients prior to participation in the study. The experimental protocol was approved by the ethics committee of Shandong University (Jinan, China).
Cell culture
The human colorectal cancer cell line SW480 was purchased from the Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured in L-15 Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), both Thermo Fisher Scientific Inc. (Waltham, MA, USA), 100 units/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in a humidified incubator at 37°C and 5% CO2.
RNA interference
Small interfering RNA (siRNA) targeting the human Lasp-1 gene and negative control (NC) siRNA were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). A total of three Lasp-1 siRNA oligonucleotides (siRNA 1, 5′-UGUAGUUCUUCAUGUUCAGUGdTdT-3′; siRNA 2, 5′-UCAAACUCCUCCUUGUAGCGCdTdT-3′; siRNA 3, 5′-AUGGUAUUUUAUGUUACUGAUdTdT-3′) and one NC siRNA (5′-CAGUCGCGUUUGCGACUGGdTdT-3′) (30 pmol per 6 wells) were used. Cells were transfected using Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h, according to the manufacturer's protocol.
Lasp-1 overexpression
The coding region of the Lasp-1 primer was synthesized by Sunshine Bio-Tech Co., Ltd. (Nanjing, China) and cloned into the pIRES2 vector (pIRES2-Lasp-1; Addgene, Cambridge, MA, USA). Cells in exponential phase were seeded in 96-well plates at a density of 3×105 cells/well and cultured for 24 h before transfection. Cells were divided into three groups: NC, siRNA and siRNA+Lasp-1. The siRNA + Lasp-1 group was included as a rescue group, and cells in this group were co-transfected with siRNA and the Lasp-1-expressing vector. A 1 µl volume of Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was diluted in 50 µl serum-free Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.) for 5 min, prior to being mixed with NC siRNA, Lasp-1 siRNA or Lasp-1 expression vector along with Lasp-1 siRNA (30 pmol per 6 wells) to prepare the transfection solution. A total of 100 µl transfection solution was added to each well and cultured for 6 h. The medium was then changed to DMEM containing 10% FBS. After 48 h of transfection, cells were collected with scraper and washed by PBS. Cells were deposited by centrifugation at 350 × g for 8 min at room temperature for further experiments.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The RNA was reverse-transcribed at 42°C for 40 min into cDNA using the Takara Reverse Transcription system kit (Takara Bio, Inc., Otsu, Japan), according to the manufacturer's protocol. qPCR was performed using the SYBR Green Premix kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the manufacturer's protocol. GAPDH was used as an internal reference gene. The following primers were used: Lasp-1, 5′-GCTTCCATTGCGAGACCTG-3′ (forward) and 5′-TGCCACTACGCTGAAACCT-3′ (reverse); ERK1/2, 5′-GTGCCGTGGAACAGGTTGT-3′ (forward) and 5′-ATGGGCTCATCACTTGGGT-3′ (reverse); and GAPDH 5′-TGTTCGTCATGGGTGTGAAC-3′ (forward) and 5′-ATGGCATGGACTGTGGTCAT-3′ (reverse). qPCR was performed on target genes under the following conditions: 95°C for 2 min, 35 cycles of 94°C for 30 sec, 58°C for 45 sec and 72°C for 35 sec. Data were collected and calculated for CT values of all samples and standards based on fluorescent quantification using GAPDH as the baseline. Standard curve was firstly plotted using CT values of standards, followed by semi-quantitative analysis by 2−ΔΔCq method (24).
Western blot analysis
Cells were lysed using the radioimmunoprecipitation assay buffer. Total protein was quantified using the Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Protein samples (50 µg) were boiled, then separated on 10% polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Merck KGaA). Membranes were blocked by 5% BSA for 1 h at room temperature and incubated overnight at 4°C with anti-Lasp-1 (1:20,000; cat. no. ab156872), anti-ERK1/2 (1:1,000; cat. no. ab17942), anti-phospho (p-)ERK1/2 (1:500; cat. no. ab214362), anti-GAPDH (1:2,000; cat. no. ab8245) primary antibodies (Abcam, Cambridge, UK) followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5,000; cat. no., ab181658, goat anti-HRP) at room temperature for 1 h (Abcam). Immunoreactive bands were detected using enhanced chemiluminescent HRP substrate (Merck KGaA).
Immunofluorescence
Cells were fixed with 4% paraformaldehyde solution for 20 min at room temperature and then washed with PBS. A solution of 0.5% TritonX-100 in PBS was used for permeabilization of cells. Blocking was performed using 10% FBS for 1 h at room temperature and cells were incubated with anti-Lasp-1, anti-ERK or anti-phospho (p-)ERK1/2 primary antibodies (1:200; cat. no. ab214362) overnight at 4°C. After washing with PBS, the cells were incubated with secondary antibody (1:500; cat. no. ab150077; goat anti-rabbit IgG H&L (Alexa Fluor® 488); Abcam). Nuclei were counterstained with DAPI for 5 min at room temperature. Staining was observed using a fluorescence microscope (×400, magnification) (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Cell proliferation assay
Cells were seeded in 96-well plates at a density of 3×104 cells/0.1 ml and were cultured for 24, 48 and 72 h. Subsequently, 10 µl Cell Counting Kit-8 reagent (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) was added to each well. After 4 h of incubation at room temperature, the optical density at 450 nm was determined. Results are reported as the mean ± standard deviation (SD) of at least three independent experiments.
Flow cytometric analysis of apoptosis
Cells transfected with the Lasp-1 or NC siRNA were harvested with trypsin-EDTA and resuspended in DMEM supplemented with 10% FBS. The cells (100 µl cell suspension, 1×106 cells/µl) were subsequently stained with fluorescein isothiocyanate-conjugated annexin V and propidium iodide (Beyotime Institute of Biotechnology, Haimen, China). Samples were incubated for 20 min in the dark at 4°C and a Cell Analyzer instrument (Merck KGaA) along with BD FACSDiva™ software (version 8.0.1; BD, Franklin Lakes, NJ, USA) was used to evaluate cellular apoptosis.
Flow cytometric analysis of cell cycle
Cells transfected with the Lasp-1 or NC siRNA were harvested with trypsin-EDTA after 72 h. Cells (1×106) were resuspended in DMEM medium containing 10% FBS. The propidium iodide/RNase staining kit (MultiSciences Biotech Co., Ltd., Hangzhou, China) was used according to the manufacturer's protocol. The proportion of cells in each phase of the cell cycle (G0/G1, S and G2/M) was determined using a flow cytometer and BD FACS Diva™ software (BD Biosciences, Franklin Lakes, NJ, USA).
Cell migration and invasion assays
Migration and invasion assays were performed using Transwell inserts (BD Biosciences). For invasion, chambers were coated with Matrigel. Cells were seeded at a density of 2×105 cells/250 µl DMEM supplemented with 0.1% FBS. Medium supplemented with 750 µl 10% FBS was added to the lower chamber and served as a chemotactic agent. Following incubation at 37°C for 12 h, non-migrating cells were discarded from the upper side of the membrane and cells on the lower side were fixed using ice-cold methanol (−20°C) and then air-dried. Cells were stained using crystal violet at 37°C for 30 min and imaged with Nikon Eclipse te200 Inverted Phase Contrast Microscopeat (×100, magnification; Nikon Corporation, Tokyo, Japan).
Statistical analysis
Results are presented as the mean ± SD. Statistical analysis was performed using SPSS software (version 19.0; IBM Corp., Armonk, NY, USA). Differences between groups were determined using analysis of variance or the Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Lasp-1 and ERK1/2 are upregulated in colorectal carcinoma
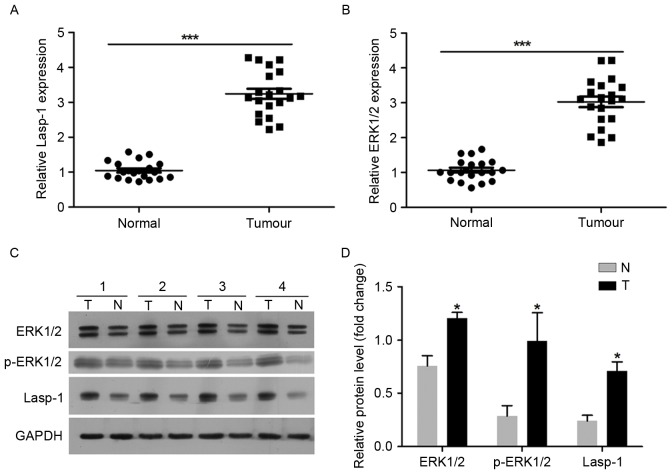
A total of 20 pairs of tumor samples (tumor, T) and adjacent normal colorectal mucosa (normal, NT) tissues were used in the present study. Lasp-1 and EKR1/2 expression levels were investigated using RT-qPCR and western blot analysis (Fig. 1). It was demonstrated that Lasp-1 mRNA expression was upregulated in tumor tissues compared with the normal controls (Fig. 1A). ERK1/2 mRNA expression was also increased in tumor tissues compared with the normal controls (Fig. 1B). Regarding the protein level, Lasp-1, EKR1/2 and p-EKR1/2 were over expressed in tumor samples (Fig. 1C and D). These results suggest that the upregulation of Lasp-1 and ERK1/2 may be associated with the progression of colorectal carcinoma.
Figure 1.
Lasp-1 and ERK1/2 expression levels in tumors and adjacent normal colorectal mucosa tissues. The mRNA expression of (A) Lasp-1 and (B) ERK1/2 was examined in 20 pairs of colorectal cancer and adjacent normal tissues using the reverse transcription-quantitative polymerase chain reaction. ***P<0.001. (C) Lasp-1, ERK1/2 and p-ERK1/2 protein expression was examined in representative samples using western blot analysis. (D) Quantitative densitometric analysis of the western blotting data (n=20; *P<0.05 vs. N). Lasp-1, LIM and SH3 protein 1; ERK, extracellular-signal-regulated kinase; p-, phospho-; N, normal group; T, tumor group.
Knockdown of Lasp-1 suppresses the expression and activation of ERK1/2 in SW480 cells
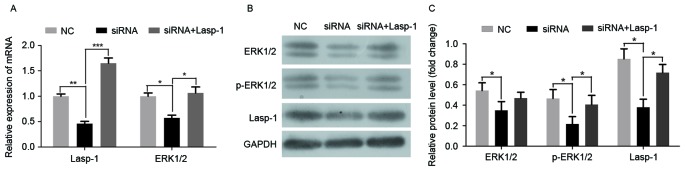
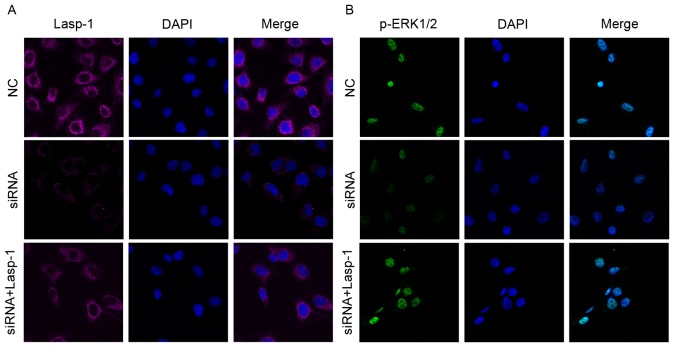
To investigate the role of Lasp-1 in the progression of colorectal carcinoma and the involved underlying molecular mechanism, Lasp-1 was knocked down by siRNA. Three siRNA oligonucleotides were designed. Among them, siRNA 1 was the most efficient (data not shown). It was then demonstrated that siRNA targeting Lasp-1 significantly downregulated Lasp-1 and ERK1/2 mRNA expression (Fig. 2A). Furthermore, the protein levels of Lasp-1, ERK1/2 and p-ERK1/2 were also suppressed by Lasp-1 knockdown (Fig. 2B and C). By contrast, Lasp-1 and p-ERK1/2 mRNA and protein levels were rescued in the siRNA group combined with Lasp-1 overexpression (Fig. 2A and C). Using immunofluorescence staining, it was further confirmed that Lasp-1 and p-ERK1/2 were decreased following Lasp-1 knockdown (Fig. 3A and B). Notably, it was observed that Lasp-1 inducedp-ERK1/2 expression, indicating that Lasp-1 regulates ERK1/2 expression in the progression of colorectal carcinoma.
Figure 2.
Lasp-1 and ERK1/2 expression in SW480 cells following Lasp-1 knockdown. (A) Lasp-1 and ERK1/2 mRNA expression determined using reverse transcription-quantitative polymerase chain reaction (n=3; *P<0.05, **P<0.01, ***P<0.001). (B) Protein expression of Lasp-1, p-ERK1/2 and ERK1/2 (n=3; *P<0.05). (C) Quantitative densitometric analysis of the western blotting data. Lasp-1, LIM and SH3 protein 1; ERK, extracellular-signal-regulated kinase; p-, phospho-; NC, negative control; siRNA, small interfering RNA.
Figure 3.
Lasp-1 and p-ERK1/2 protein expression following Lasp-1 knockdown. (A) Lasp-1 protein expression detected using immunofluorescence. The Merge panels are superimposed images of Lasp-1 staining in red and nuclei staining in blue (using DAPI). Magnification, ×400. (B) p-ERK1/2 protein expression detected using immunofluorescence. The Merge panels are superimposed images of p-ERK1/2 staining in green and nuclei staining in blue (using DAPI). Magnification, ×400. Lasp-1, LIM and SH3 protein 1; ERK, extracellular-signal-regulated kinase; p-, phospho-; NC, negative control; siRNA, small interfering RNA.
Lasp-1 knockdown inhibits cellular proliferation, induces apoptosis and cell cycle arrest in the G0/G1 phase
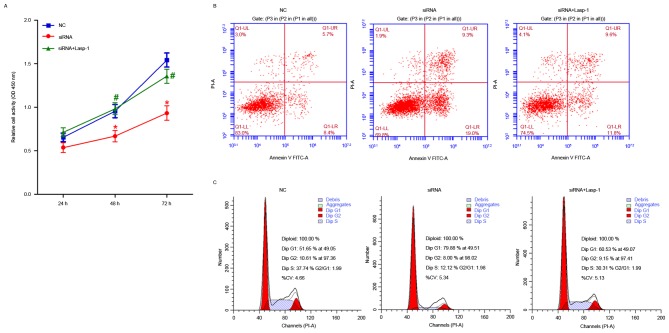
The effects of Lasp-1 knockdown on cellular proliferation, induction of apoptosis and cell cycle arrest were investigated (Fig. 4). Cellular proliferation was impaired at 48 and 72 h after Lasp-1 knockdown (Fig. 4A). Furthermore, increased early-(19.0 vs. 8.4%) as well as late-(9.3 vs. 5.7%) stage (Fig. 4B) apoptotic cell death was detected. Furthermore, Lasp-1 silencing increased the proportion of cells in the G0/G1 phase of the cell cycle (79.88 vs. 51.65%) and decreased the proportion of cells in the S phase (12.12 vs. 37.74%). By contrast, overexpression of Lasp-1induced cellular proliferation and inhibited the induction of apoptotic cell death, compared with cells treated with siRNA (Fig. 4B and C). These results indicate that cell-cycle arrest in the G0/G1 phase of the cell cycle was induced following Lasp-1 knockdown.
Figure 4.
Effects of Lasp-1 in cellular proliferation, induction of apoptotic cell death and cell cycle progression in colorectal carcinoma cells. Cells were transfected with Lasp-1 siRNA or NC siRNA. (A) Cellular proliferation was determined using the Cell Counting Kit-8 assay (n=3; *P<0.05 vs. NC; #P<0.05 vs. siRNA). (B) Cellular apoptosis and (C) cell cycle progression were detected using flow cytometric analysis. Lasp-1, LIM and SH3 protein 1; NC, negative control; siRNA, small interfering RNA; PI, propidium iodide; FITC, fluorescein isothiocyanate.
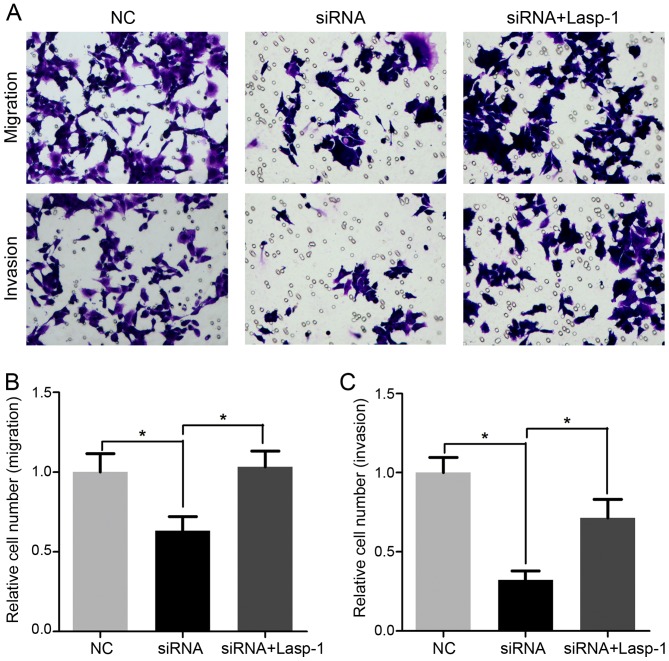
Decreased Lasp-1 expression inhibits cellular migration and invasion
Cell migration and invasion following Lasp-1 knockdown were investigated using the Transwell assay (Fig. 5). It was demonstrated that cellular migration and invasion were significantly inhibited in cells treated with Lasp-1 siRNA compared with the NC group (Fig. 5B and C). Notably, migration and invasion were increased following Lasp-1 overexpression. These results indicated that Lasp-1 promotes metastasis in colorectal carcinoma cells.
Figure 5.
Effects of Lasp-1 in cellular migration and invasion. (A) Cellular migration and invasion were detected following a 48-h transfection period with Lasp-1 siRNA or siRNA and Lasp-1 overexpression using a Transwell assay. Magnification, ×400. Quantification of the (B) cell migration and (C) invasion data (n=3; *P<0.05). Lasp-1, LIM and SH3 protein 1; NC, negative control; siRNA, small interfering RNA.
Discussion
Colorectal carcinoma is the second leading cause of cancer-associated mortality worldwide (1,2). The results of the present study suggest that Lasp-1 regulates the progression of colorectal carcinoma via activation of the MAPK signaling pathway. The Lasp-1 contains a N-terminal LIM domain, composed of two sequential zinc-binding modules with a typical LIM motif, followed by a C-terminal Src homology region 3 (SH3) domain and by tandem 35-residue nebulin-like repeats R1 and R2 (14,25). The SH3 domain is shared with diverse structural and signaling proteins (26). It has been suggested that LIM domains bound to DNA are involved in homodimerization (27). The R1 and R2 repeats are involved in protein-protein interactions and in cytoskeleton stability (28,29). Lasp-1 protein interaction is essential for cell motility, resulting in the activation of ERK1/2 (30). In the present study, it was demonstrated that Lasp-1 and ERK1/2 were upregulated in tumor tissues compared with adjacent normal colorectal mucosa tissues in 20 patients with colorectal carcinoma. Furthermore, decreased Lasp-1 expression was associated with decreased ERK1/2 levels.
Lasp-1 phosphorylates a number of proteins involved in the MAPK signaling pathway (20). It has been demonstrated that miR-133α downregulated the expression of Lasp-1, by inhibiting the phosphorylation of ERK and MEK, resulting in suppressed tumor growth and metastasis in liver and lung cancer cells (22). Exogenous miR-133α inhibited the MAPK signaling pathway and increased the expression of epithelial markers (23). A similar effect on epithelial-mesenchymal transition has been observed following Lasp-1 targeting (22). Consistently, the results of the present study demonstrated that Lasp-1 mRNA was upregulated in tumor samples, compared with adjacent normal tissues. Additionally, Lasp-1 knockdown induced apoptotic cell death and cell cycle arrest in the G0/G1 phase. Furthermore, it suppressed proliferation, migration and invasion, suggesting a negative regulation of the progression and metastatic potential of colorectal carcinoma.
Notably, Lasp-1 promotes proliferation and metastasis in gallbladder cancer via the induction of cell cycle arrest at the G2/M phase. Therefore, it is concluded that Lasp-1 may promote cancer cell proliferation and metastasis via modulating different phases of the cell cycle. A number of key factors, including cyclin-dependent kinase, mitosis-promoting factor, ataxia telangiectasia mutated serine/threonine kinase, p53 and retinoblastoma are involved in the regulation of cell cycle progression (31–33). Additional studies are required to validate the effect of Lasp-1 in a variety of tumors and investigate the role of ERK1/2 and other MAPK-associated signaling pathways, including the stress-activated protein kinase/c-Jun N-terminal kinase signaling pathway and the p38 MAPK signaling pathway in tumor progression.
The results of the present study demonstrated that the role of Lasp-1 in the progression and metastasis of colorectal carcinoma is associated with the MAPK signaling pathway. The data of the present study elucidate the effect of Lasp-1 in the progression and metastasis of colorectal carcinoma and suggest a novel potential target for the treatment of patients with metastatic colorectal carcinoma.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Quintero E, Carrillo M, Leoz ML, Cubiella J, Gargallo C, Lanas A, Bujanda L, Gimeno-García AZ, Hernández-Guerra M, Nicolás-Pérez D, et al. Risk of advanced neoplasia in first-degree relatives with colorectal cancer: A large multicenter cross-sectional study. PLoS Med. 2016;13:e1002008. doi: 10.1371/journal.pmed.1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krbal L, Hanušová V, Soukup J, John S, Matoušková P, Ryška A. Contribution of in vitro comparison of colorectal carcinoma cells from primary and metastatic lesions to elucidation of mechanisms of tumor progression and response to anticancer therapy. Tumour Biol. 2016;37:9565–9578. doi: 10.1007/s13277-016-4839-y. [DOI] [PubMed] [Google Scholar]
- 5.Tan WJ, Chew MH, Tan IB, Law JH, Zhao R, Acharyya S, Mao YL, Fernandez LG, Loi CT, Tang CL. Palliative surgical intervention in metastatic colorectal carcinoma: A prospective analysis of quality of life. Colorectal Dis. 2016;18:357–363. doi: 10.1111/codi.13142. [DOI] [PubMed] [Google Scholar]
- 6.Grunewald TG, Kammerer U, Schulze E, Schindler D, Honig A, Zimmer M, Butt E. Silencing of LASP-1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp Cell Res. 2006;312:974–982. doi: 10.1016/j.yexcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Vaman VSA, Poppe H, Houben R, Grunewald TG, Goebeler M, Butt E. LASP1, a newly identified melanocytic protein with a possible role in melanin release, but not in melanoma progression. PLoS One. 2015;10:e0129219. doi: 10.1371/journal.pone.0129219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang F, Zhou X, Du S, Zhao Y, Ren W, Deng Q, Wang F, Yuan J. LIM and SH3 domain protein 1 (LASP-1) overexpression was associated with aggressive phenotype and poor prognosis in clear cell renal cell cancer. PLoS One. 2014;9:e100557. doi: 10.1371/journal.pone.0100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng J, Wang F, Lu S, Wang X. LASP-1, regulated by miR-203, promotes tumor proliferation and aggressiveness in human non-small cell lung cancer. Exp Mol Pathol. 2016;100:116–124. doi: 10.1016/j.yexmp.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Hailer A, Grunewald TG, Orth M, Reiss C, Kneitz B, Spahn M, Butt E. Loss of tumor suppressor mir-203 mediates overexpression of LIM and SH3 protein 1 (LASP1) in high-risk prostate cancer thereby increasing cell proliferation and migration. Oncotarget. 2014;5:4144–4153. doi: 10.18632/oncotarget.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Chen Y, Wang X, Zhang H, Zhang Y, Gao Y, Weng M, Wang L, Liang H, Li M, et al. LASP-1 induces proliferation, metastasis and cell cycle arrest at the G2/M phase in gallbladder cancer by down-regulating S100P via the PI3K/AKT pathway. Cancer Lett. 2016;372:239–250. doi: 10.1016/j.canlet.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Payton S. Bladder cancer: LASP-1-a promising urine marker for detection of bladder cancer. Nat Rev Urol. 2012;9:240. doi: 10.1038/nrurol.2012.84. [DOI] [PubMed] [Google Scholar]
- 13.Ardelt P, Grünemay N, Strehl A, Jilg C, Miernik A, Kneitz B, Butt E. LASP-1, a novel urinary marker for detection of bladder cancer. Urol Oncol. 2013;31:1591–1598. doi: 10.1016/j.urolonc.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Orth MF, Cazes A, Butt E, Grunewald TG. An update on the LIM and SH3 domain protein 1 (LASP1): A versatile structural, signaling, and biomarker protein. Oncotarget. 2015;6:26–42. doi: 10.18632/oncotarget.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvi A, Bongarzone I, Ferrari L, Abeni E, Arici B, De Bortoli M, Scuri S, Bonini D, Grossi I, Benetti A, et al. Molecular characterization of LASP-1 expression reveals vimentin as its new partner in human hepatocellular carcinoma cells. Int J Oncol. 2015;46:1901–1912. doi: 10.3892/ijo.2015.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Li W, Jin X, Cui S, Zhao L. LIM and SH3 protein 1, a promoter of cell proliferation and migration, is a novel independent prognostic indicator in hepatocellular carcinoma. Eur J Cancer. 2013;49:974–983. doi: 10.1016/j.ejca.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Wang H, Liu C, Liu Y, Wang X, Wang S, Sun X, Li J, Deng Y, Jiang Y, Ding Y. Promotion of colorectal cancer growth and metastasis by the LIM and SH3 domain protein 1. Gut. 2010;59:1226–1235. doi: 10.1136/gut.2009.202739. [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Wang H, Sun X, Ding Y. Comparative proteomic analysis identifies proteins associated with the development and progression of colorectal carcinoma. FEBS J. 2010;277:4195–4204. doi: 10.1111/j.1742-4658.2010.07808.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Li Z, Chu B, Zhang F, Zhang Y, Ke F, Chen Y, Xu Y, Liu S, Zhao S, et al. Upregulated LASP-1 correlates with a malignant phenotype and its potential therapeutic role in human cholangiocarcinoma. Tumour Biol. 2016;37:8305–8315. doi: 10.1007/s13277-015-4704-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Shi J, Luo Y, Liao Q, Niu Y, Zhang F, Shao Z, Ding Y, Zhao L. LIM and SH3 protein 1 induces TGFβ-mediated epithelial-mesenchymal transition in human colorectal cancer by regulating S100A4 expression. Clin Cancer Res. 2014;20:5835–5847. doi: 10.1158/1078-0432.CCR-14-0485. [DOI] [PubMed] [Google Scholar]
- 21.Lin YH, Park ZY, Lin D, Brahmbhatt AA, Rio MC, Yates JR, III, Klemke RL. Regulation of cell migration and survival by focal adhesion targeting of Lasp-1. J Cell Biol. 2004;165:421–432. doi: 10.1083/jcb.200311045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, An H, Wang B, Liao Q, Li W, Jin X, Cui S, Zhang Y, Ding Y, Zhao L. miR-133a represses tumour growth and metastasis in colorectal cancer by targeting LIM and SH3 protein 1 and inhibiting the MAPK pathway. Eur J Cancer. 2013;49:3924–3935. doi: 10.1016/j.ejca.2013.07.149. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Zhang Y, Wang H, Zhang G, Ding Y, Zhao L. Tumor suppressor miR-1 restrains epithelial-mesenchymal transition and metastasis of colorectal carcinoma via the MAPK and PI3K/AKT pathway. J Transl Med. 2014;12:244. doi: 10.1186/s12967-014-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Tomasetto C, Moog-Lutz C, Régnier CH, Schreiber V, Basset P, Rio MC. Lasp-1 (MLN 50) defines a new LIM protein subfamily characterized by the association of LIM and SH3 domains. FEBS Lett. 1995;373:245–249. doi: 10.1016/0014-5793(95)01040-L. [DOI] [PubMed] [Google Scholar]
- 26.Kay BK. SH3 domains come of age. FEBS Lett. 2012;586:2606–2608. doi: 10.1016/j.febslet.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammarström A, Berndt KD, Sillard R, Adermann K, Otting G. Solution structure of a naturally-occurring zinc-peptide complex demonstrates that the N-terminal zinc-binding module of the Lasp-1 LIM domain is an independent folding unit. Biochemistry. 1996;35:12723–12732. doi: 10.1021/bi961149j. [DOI] [PubMed] [Google Scholar]
- 28.Pappas CT, Bliss KT, Zieseniss A, Gregorio CC. The Nebulin family: An actin support group. Trends Cell Biol. 2011;21:29–37. doi: 10.1016/j.tcb.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mihlan S, Reiß C, Thalheimer P, Herterich S, Gaetzner S, Kremerskothen J, Pavenstädt HJ, Lewandrowski U, Sickmann A, Butt E. Nuclear import of LASP-1 is regulated by phosphorylation and dynamic protein-protein interactions. Oncogene. 2013;32:2107–2113. doi: 10.1038/onc.2012.216. [DOI] [PubMed] [Google Scholar]
- 30.Raman D, Sai J, Neel NF, Chew CS, Richmond A. LIM and SH3 protein-1 modulates CXCR2-mediated cell migration. PLoS One. 2010;5:e10050. doi: 10.1371/journal.pone.0010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celeghini C, Voltan R, Rimondi E, Gattei V, Zauli G. Perifosine selectively induces cell cycle block and modulates retinoblastoma and E2F1 protein levels in p53 mutated leukemic cell lines. Invest New Drugs. 2011;29:392–395. doi: 10.1007/s10637-009-9370-1. [DOI] [PubMed] [Google Scholar]
- 32.Park SJ, Yang SW, Kim BC. Transforming growth factor-β1 induces cell cycle arrest by activating atypical cyclin-dependent kinase 5 through up-regulation of Smad3-dependent p35 expression in human MCF10A mammary epithelial cells. Biochem Biophys Res Commun. 2016;472:502–507. doi: 10.1016/j.bbrc.2016.02.121. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhary P, Sharma R, Sahu M, Vishwanatha JK, Awasthi S, Awasthi YC. 4-Hydroxynonenal induces G2/M phase cell cycle arrest by activation of the ataxia telangiectasia mutated and Rad3-related protein (ATR)/checkpoint kinase 1 (Chk1) signaling pathway. J Biol Chem. 2013;288:20532–20546. doi: 10.1074/jbc.M113.467662. [DOI] [PMC free article] [PubMed] [Google Scholar]