Figure 8. Competitive stimulation of yeast Hsp90 (Hsc82) ATPase by Δ131Δ and designed compounds.
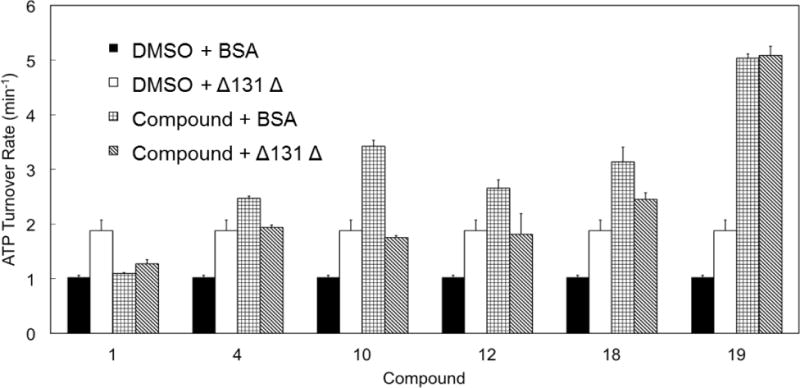
Hsc82 ATPase is measured in the presence of Δ131Δ (50 μM) or selected allosteric activators (50 μM, 1, 4, 10, 12, 18, 19) or the combination of both. Reactions with BSA and DMSO serve as negative control. Δ131Δ stimulated Hsc82 ATP-hydrolysis by 1.8 fold. The stimulation by compounds alone varies with each compound. However, when the chaperone was stimulated by both Δ131Δ and designed accelerators, the resultant ATPase rates mostly fell in the range of the rate yielded by Δ131Δ or compounds stimulation alone.
