Fig. 4.
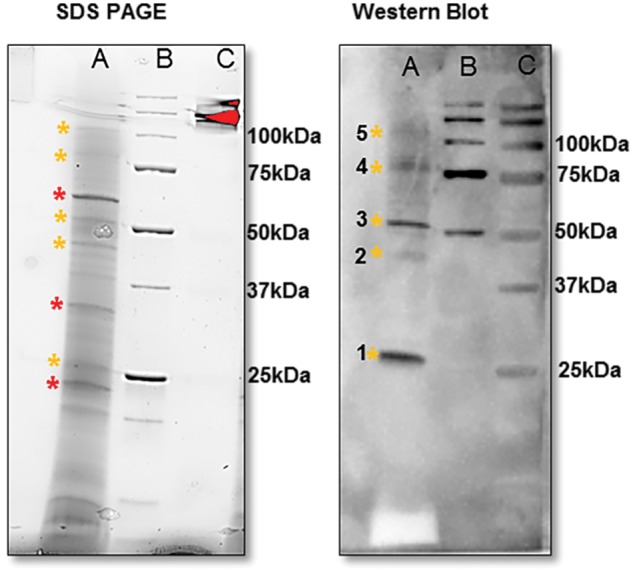
Mature VpVAN homodimer and putative oligomers detected in the crude V. planifolia extracts as demonstrated by SDS–PAGE followed by Western blot analysis. Lane A: crude protein extract from a 7-month-old vanilla pod from V. planifolia. Lane B: pre-stained Bio-Rad protein ladder. Lane C: unstained Bio-Rad protein ladder. 1, VpVAN mature protein (25 kDa); 2, VpVAN immature protein (39 kDa); 3, putative mature VpVAN homodimer (50 kDa); 4, putative mature VpVAN trimer (75 kDa); 5, putative mature VpVAN oligomers (100 kDa). The proteins present in a 7-month-old vanilla pod were separated by SDS–PAGE (12% Criterion™ TGX Stain-Free Precast Gels) and visualized using the ChemiDoc MP Imaging System (Bio-Rad). The major protein components present in the pod extracts are marked with * (red) while VpVAN and putative oligomers are marked with * (yellow). Western blot analysis was conducted using a C-terminal sequence-specific antibody against VpVAN in a 1 : 50 dilution. At this extremely high antibody concentration, the primary antibody demonstrated specificity towards VpVAN. No cross-reactions were observed with the major protein components present in the extract.
