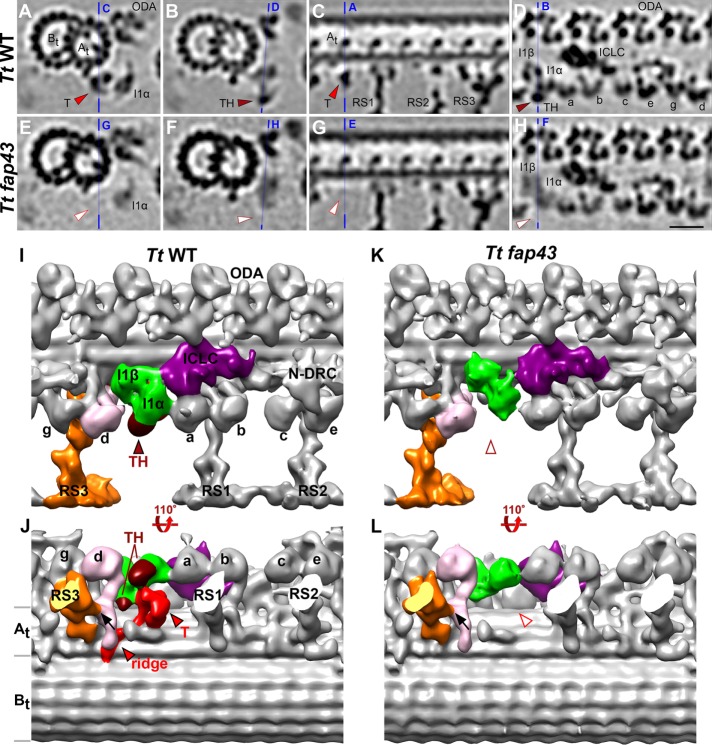
FIGURE 1:
Comparison between wild-type and T/TH mutant axonemes to define the three-dimensional structure of the I1-associated T/TH complex. (A–H) Tomographic slices of the averaged 96-nm-long axonemal repeats of wild-type Tetrahymena thermophila (A–D) and its fap43 knockout mutant (E–H) viewed in cross-sectional (A, B, E, and F) and longitudinal (C, D, G, and H) orientations. Note that all cross-sections in the paper are shown viewed from proximal toward the ciliary tip, and in all longitudinal views proximal is on the left, unless otherwise noted. Blue lines indicate the locations of the slices in the respective panels. Electron densities corresponding to tether (T, red arrowheads in A and C) and tether head (TH, dark red arrowheads in B and D) were absent from the fap43 knockout mutant axonemes (white arrowheads in E–H). (I–L) Isosurface renderings show the three-dimensional structures of the averaged axonemal repeat of wild type and the fap43 mutant in front (I, K) and bottom (J, L) view. The entire I1 dynein complex (I1α and I1β motor domains, green; intermediate and light chain complex (ICLC), purple) was observed in the fap43 mutant, whereas the tether (red) and tether head (dark red) were completely missing. Black arrows in J and L indicate the connection between inner dynein arm d (IDA d, rose) and radial spoke 3 (RS3, orange). Other labels: At and Bt, A- and B-tubule; a–e and g, inner dynein arm isoforms; N-DRC, nexin dynein regulatory complex; ODA, outer dynein arm. Scale bar: 20 nm (valid for A–H).