FIGURE 5:
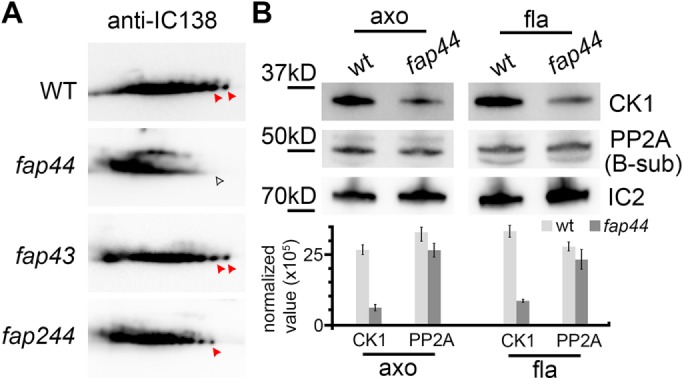
Biochemical studies show IC138 hyperphosphorylation and CK1 reduction in the C. reinhardtii fap44 mutant. (A) Two-dimensional gel immunoblots of axonemal proteins extracted from Chlamydomonas wild type and fap44, fap43, and fap244 mutants probed with anti-IC138. The phosphorylation level of IC138 is higher on the acidic side of the gel (left side) than the basic side (King and Dutcher, 1997). Red arrowheads indicate non- or low-phosphorylated isoforms of IC138, which were reduced in fap44 (white arrowhead). (B) Immunoblots (top) of axonemal (axo) and flagellar (fla) proteins extracted from Chlamydomonas wild type and fap44, and relative densitometry quantification of the bands (bottom) normalized to IC2 show reduced abundance of casein kinase 1 (CK1) but not of phosphatase 2A (PP2A) in fap44. Blots were probed with anti-CK1, anti-PP2A (B-subunit), and anti-IC2 (control). Results represent mean±SD (n = 3).
