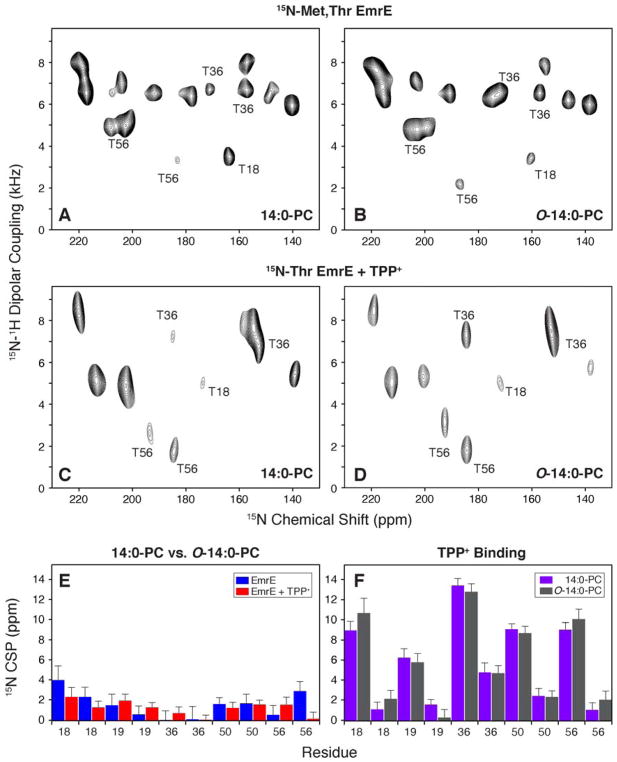
Figure 5.
1H/15N PISEMA spectra of EmrE reconstituted in bicelles consisting of 14:0-PC/6:0-PC or O-14:0-PC/6:0-PC. For simplicity, the panels are labeled by the long chain lipid (either 14:0-PC or O-14:0-PC). (A, B) [15N-Thr, 15N-Met] labeled EmrE in magnetically aligned bicelles in the drug-free, protonated form of EmrE at pH 5.8. (C, D) [15N-Thr] labeled EmrE in magnetically aligned bicelles in the TPP+ bound form of the protein. (E) 15N chemical shift perturbations (CSP) between drug-free EmrE in 14:0-PC and O-14:0-PC bicelles is shown in blue. Similarly the TPP+ bound form is compared between 14:0-PC and O-14:0-PC bicelles and is shown in red. (F) A comparison of 15N CSP values for TPP+ binding to EmrE. Purple bars show the perturbations induced to EmrE within the 14:0-PC bicelle and gray bars correspond to CSPs calculated in O-14:0-PC bicelles. Panels E and F plot the threonine residues in EmrE. Thr18 and Thr19 are located in TM1, Thr36 and Thr50 are located in TM2, and Thr56 is located in the loop between TM2 and TM3.