Abstract
Importance
Midlife vascular risk factors have been associated with late-life dementia; whether these risk factors directly contribute to brain amyloid deposition is less well understood.
Objective
To determine if midlife vascular risk factors are associated with late-life brain amyloid deposition, measured using florbetapir positron emission tomography (PET).
Design, Setting, and Participants
Prospective cohort study in 3 U.S. communities (Washington County, MD; Forsyth County, NC; and Jackson, MS). 346 participants without dementia in the Atherosclerosis Risk in Communities (ARIC)-PET Amyloid Imaging Study; evaluation of vascular risk factors and markers since 1987-1989, with florbetapir PET scans (2011-2013).
Exposures
Vascular risk factors at ARIC baseline (ages 45-64) were evaluated in multivariable models including age, sex, race, APOE genotype, and educational level.
Outcome
Standardized Uptake Value Ratios (SUVR) were calculated from PET scans; a mean global cortical SUVR was calculated. Elevated florbetapir (defined at SUVR>1.2) was the dependent variable.
Results
In 322 participants without dementia and with nonmissing midlife vascular risk factors at baseline (43% black, 58% female, mean age 52), SUVR (positive in 164 (50.9%) of participants) was measured >20 years later (median followup 23.5; IQR 23.0-24.3) when participants were 67-88 (mean 76 y). Elevated body mass index midlife (BMI) was associated with elevated SUVR (OR 2.06, 95% CI 1.16, 3.65). At baseline, 65 participants had no vascular risk factors, 123 had one, and 134 had two or more; a higher number of midlife risk factors was associated with elevated amyloid SUVR at followup, 30.8% (n=20), 50.4% (n=62), and 61.2% (n=82), respectively. In adjusted models, compared to 0 midlife vascular risk factors, the odds ratio for elevated SUVR associated with 1 vascular risk factor was 1.88 (95% CI 0.95-3.72) and was 2.88 (95% CI 1.46-5.69) for 2 or more vascular risk factors No significant race by risk factor interactions were found. Late-life vascular risk factors were not associated with late-life brain amyloid: (2 or more late-life vascular risk factors compared to 0: OR 1.66, 95% CI 0.75-3.69).
Conclusions and Relevance
An increasing number of midlife vascular risk factors was significantly associated with elevated amyloid SUVR; this association was not significant for late-life risk factors. These findings are consistent with a role of vascular disease in the development of Alzheimer’s Disease.
Keywords: dementia, amyloid, hypertension, epidemiology
Introduction
Increasing evidence supports a role of vascular risk factors and markers in the development and etiology of Alzheimer’s Disease (AD). Most major vascular risk factors, including hypertension,1–4 diabetes,5 smoking,3,6 and hypercholesterolemia,4,7 particularly when measured in midlife, have been associated with risk of dementia generally, and AD specifically. Whether these risk factors directly increase the neurodegeneration specifically associated with AD (such as through increasing amyloid deposition), or lead to other cerebral changes that, in conjunction with ongoing neurodegeneration, might worsen cognitive performance is not yet known.
The role of the APOE ε4 allele as a genetic risk factor for AD is well established,8 but its role as a modifier of the relationship between vascular disease and AD is less well understood. APOE ε4 carriage in combination with vascular disease may work synergistically to increase risk for AD9, with worse cognitive outcomes in persons with both an increased genetic and vascular risk. These relationships are further complicated by the direct association between APOE and vascular disease: the ε4 allele is proatherogenic,10 so evaluation of the interactive effects of APOE and vascular risk factors on AD neuropathology must also consider the independent contribution of APOE to vascular disease.
The availability of imaging biomarkers for brain amyloid allows the study of individuals before the development of dementia, and thereby allows consideration of the relative contributions of vascular disease and amyloid to cognition, as well as the contribution of vascular disease to amyloid deposition. In this study, vascular risk factors were collected for over 25 years in participants from the Atherosclerosis Risk in Communities (ARIC) Study, with brain amyloid PET imaging obtained in late-life, in order to evaluate the associations between vascular risk factors, APOE genotype, and brain amyloid deposition.
Methods
The ARIC-PET Study is an ancillary to the ongoing ARIC-Neurocognitive study (ARIC-NCS), which itself is a major ancillary of the ARIC study. The study was approved by each institution’s institutional review board. All participants provided written informed consent.
Participant inclusion
Participants underwent a baseline visit in 1987-1989, when 15,792 individuals were recruited from four US communities,11 with four additional in-person visits, most recently in 2011-2013, and with annual/semi-annual telephone calls during the study duration. Each visit (including baseline) has included in-person assessment of vascular risk factors, with a shorter cognitive evaluation at the 2nd and 4th visits, and a more extensive neuropsychological assessment including informant interview at visit 5 (2011-2013).
Nearly 2000 participants without contraindication to MRI were invited for brain MRI, based on any of the following12: (1) prior research brain MRI as part of the cohort; (2) low cognitive scores or cognitive decline at visit 5; (3) a random age-stratified sample of cognitively normal participants. This study recruited from this subset, with the additional exclusion criteria of heavy current alcohol use, renal dysfunction, prolonged (>450 msec) QT-c interval, or neuropsychological results consistent with dementia. Methods used to adjudicate mild cognitive impairment (MCI) and dementia research diagnoses are described elsewhere,13 but for the purposes of exclusion for this study, participants were excluded if they had an already-adjudicated research diagnosis of dementia, a Clinical Dementia Rating sum-of-boxes score >3, a Functional Activities Questionnaire score >5, or a Mini-Mental State Examination <19(Blacks) or <21(whites).13
Brain MRI and PET
Brain MRI scans, obtained as research studies at a 3T MRI facility near each field center, were read centrally at the Mayo Clinic.12 PET images were co-registered using MP-RAGE sequences.
The details of PET image processing and coregistration with MRI, carried out at the JHU reading center, were described previously.14 A global cortical measure of florbetapir uptake was used as a weighted average (based on ROI size) of the orbitofrontal, prefrontal, and superior frontal cortices, the lateral temporal, parietal, and occipital lobes, the precuneus, anterior cingulate, and posterior cingulate. An automated region for cerebellum gray was used as reference.14–17 SUVR values were dichotomized at the sample median of SUVR>1.214, although other cutpoints (1.1118 and 1.1019) were explored in sensitivity analyses. PET scans were obtained within 1 year of MRI scans, although ideally within 6 months.
Vascular risk factors and other covariates
Vascular risk factors were evaluated at all in-person visits. Analyses in this study focused on risk factor status in midlife (visit 1, ages 45-64 years) as well as concurrent to PET (visit 5, ages 69-88). For demographic factors, date of birth, queried at visit 1, was used to calculate age at each visit, and sex and educational level were self-reported from visit 1. Race was self-selected out of several fixed categories (Asian, Black, American Indian/Alaskan Indian, or White); race was evaluated given previously reported differences in dementia rates,23 and baseline differences in amyloid SUVR in this study.14 Blood pressure (BP) was measured two to three times per visit; hypertension was present if the mean of the last two measurements was >140 mm Hg (systolic) or >90 mm Hg (diastolic), or if the participant was on an antihypertensive medication at that visit. Diabetes was defined as fasting glucose≥126 mg/dL, non-fasting glucose≥200 mg/dL, self-report of physician-diagnosed diabetes, or use of oral diabetes medications or insulin. Estimated 10-year stroke risk, calculated at visits 1 and 5, was based on a previously published algorithm for stroke risk,20 and smoking history was self-reported as current/former/never. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). Fasting lipids were measured at each visit;21 plasma total cholesterol was measured using enzymatic methods.22 APOE was genotyped previously, and defined based on the number of ε4 alleles (0,1, or 2).
To evaluate cumulative burden of vascular risk factors, the number of vascular risk factors present in midlife (visit 1) and late-life (visit 5) was tallied, up to 5 maximum, including: current smoking; hypertension; diabetes; obesity (BMI ≥30 kg/m2); and elevated total cholesterol (≥200 mg/dL). Because very few individuals had 3 or 4 risk factors, and none who were included in our study had 5 risk factors, the number of midlife risk factors was categorized into 0, 1, or ≥2.
Statistical analysis
Stata SE version 13 for Macintosh (College Station, TX) was used for all analyses. Group comparisons were evaluated visually and with descriptive statistics. Florbetapir uptake was evaluated as a dichotomous (SUVR>1.2) measure, in logistic regression models; additional cutpoints were evaluated in sensitivity analyses. Due to the highly skewed florbetapir SUVR variable, regression analyses did not evaluate continuous florbetapir uptake. Multivariable logistic regression models evaluating the described vascular risk factors, in the same model, included age, sex, race, educational level, and APOE status; model goodness-of-fit was confirmed with Hosmer-Lemeshow test (p=0.27). Models had low residuals and were without evidence of high leverage points. The same analyses were then repeated but using a single variable for the number of vascular risk factors (midlife or late-life, in separate models). Based on a priori hypotheses, models were then stratified by race, sex, and also by APOE status (1 or 2 ε4 alleles versus no ε4 alleles), with formal tests for interaction by race, sex, and by APOE. P-values of <0.05 were considered statistically significant; testing was 2-sided.
Sensitivity analyses stratified the sample by cognitive status, with separate analysis of individuals with normal cognition or with MCI, in addition to the evaluation of other florbetapir cutpoints. In addition, multiple imputation was used for those 21 individuals excluded due to missing covariate data, as a sensitivity analysis. Amyloid deposition in distinct regions of interest was also considered.
Results
Participants had a mean age of 52 at the time of midlife vascular risk factor assessment, and 76 at the time of PET imaging; median followup was 23.5 years (IQR 23.0-24.3 years). Fifty-seven percent of the sample was white, with 43% being of black race (Table 1). Rates of hypertension and diabetes increased from midlife to late-life (Table 1), as did BMI and the stroke risk score, and lipid levels and smoking rates decreased. No participants had five vascular risk factors either at visit 1 or 5, and the number of risk factors was greater at visit 5 than at visit 1. At visit 1, 65 participants (20%) had no risk factors, 123 (38%) had one risk factor, and 134 (42%) had 2 or more risk factors. Of the 346 participants who underwent florbetapir PET imaging, 24 total were excluded, leaving 322 participants for analysis (supplemental figure, supplemental table 1). Defined as positive above the sample median, 164 (50.9%) of the cohort had an elevated amyloid SUVR.
Table 1.
Participant characteristics overall and by race, N=322.
| ARIC Visit 1 (1987-1989) | ARIC Visit 5 (2011-2013) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall (N=322) |
Blacks (n=139) |
Whites (n=183) |
Overall (N=322) |
Blacks (n=139) |
Whites (n=183) |
|
| Age (years), mean (SD) | 52.2 (5.2) | 52.0 (5.1) | 52.4 (5.3) | 75.8 (5.3) | 75.6 (5.2) | 76.0 (5.5) |
| Female, No. (%) | 185 (57.5) | 85 (61.2) | 100 (54.6) | 185 (57.5) | 85 (61.2) | 100 (54.6) |
| Education level, No. (%) | ||||||
| College, graduate, or professional school | 132 (41.0) | 60 (43.2) | 72 (39.3) | 132 (41.0) | 60 (43.2) | 72 (39.3) |
| High school, GED, or vocational school | 139 (43.2) | 50 (36.0) | 89 (48.6) | 139 (43.2) | 50 (36.0) | 89 (48.6) |
| < High school | 51 (15.8) | 29 (20.9) | 22 (12.0) | 51 (15.8) | 29 (20.9) | 22 (12.0) |
| APOE ε4 genotype, No. (%) | ||||||
| 2/2 or 2/3 or 3/3 | 222 (68.9) | 88 (63.3) | 134 (73.2) | 222 (68.9) | 88 (63.3) | 134 (73.2) |
| 2/4 or 3/4 | 92 (28.6) | 46 (33.1) | 46 (25.1) | 92 (28.6) | 46 (33.1) | 46 (25.1) |
| 4/4 | 8 (2.5) | 5 (3.6) | 3 (1.6) | 8 (2.5) | 5 (3.6) | 3 (1.6) |
| Body mass index (kg/m2), mean (SD) | 27.4 (4.4) | 28.4 (4.5) | 26.7 (4.1) | 29.0 (5.3) | 29.8 (5.3) | 28.4 (5.1) |
| Body mass index ≥30 kg/m2, No (%) | 83 (25.8) | 45 (32.4) | 38 (20.8) | 121 (37.6) | 62 (44.6) | 59 (32.2) |
| Current Smoking, No. (%) | 55 (17.1) | 24 (17.3) | 31 (16.9) | 16 (5.0) | 8 (5.8) | 8 (4.4) |
| Hypertension, No. (%) | 95 (29.5) | 58 (41.7) | 37 (20.2) | 230 (71.4) | 114 (82.0) | 116 (63.4) |
| Diabetes, No. (%) | 20 (6.2) | 7 (5.0) | 13 (7.1) | 130 (40.4) | 55 (39.6) | 75 (41.0) |
| Total cholesterol (mg/dl), mean (SD) | 208.3 (39.0) | 211.3 (41.9) | 206.0 (36.6) | 180.8 (39.6) | 185.1 (42.2) | 177.5 (37.4) |
| Total cholesterol ≥200 mg/dl, No. (%) | 180 (55.9) | 79 (56.8) | 101 (55.2) | 94 (29.2) | 47 (33.8) | 47 (25.7) |
| 10-year stroke risk score* (%), median (IQR) | 1.0 (0.6, 1.7) | 1.4 (0.8, 2.4) | 0.8 (0.5, 1.3) | 11.9 (5.3, 22.6) | 14.0 (7.2, 27.4) | 8.6 (4.0, 17.9) |
| Number of vascular risk factors**, No. (%) | ||||||
| 0 vascular risk factors | 65 (20.2) | 22 (15.8) | 43 (23.5) | 35 (10.9) | 6 (4.3) | 29 (15.9) |
| 1 vascular risk factor | 123 (38.2) | 46 (33.1) | 77 (42.1) | 82 (25.5) | 30 (21.6) | 52 (28.4) |
| 2 vascular risk factors | 93 (28.9) | 46 (33.1) | 47 (25.7) | 116 (36.0) | 57 (41.0) | 59 (32.2) |
| 3 vascular risk factors | 40 (12.4) | 25 (18.0) | 15 (8.2) | 79 (24.5) | 42 (30.2) | 37 (20.2) |
| 4 vascular risk factors | 1 (0.3) | 0 (0.0) | 1 (0.6) | 10 (3.1) | 4 (2.9) | 6 (3.3) |
| 5 vascular risk factors | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mild cognitive impairment, No. (%) | – | – | – | 87 (27.0) | 37 (26.6) | 48 (27.3) |
10-year stroke risk score visit 1 N=305 (n=134 blacks and n=171 whites) with non-missing data. 10-year stroke risk score visit 5 N=304 (n=131 blacks and n=173 whites) with non-missing data. 10-year stroke risk score includes race, smoking status, age, prior coronary heart disease, antihypertensive medication use, left ventricular hypertrophy, diabetes status, systolic blood pressure, and sex.20
Vascular risk factors included: body mass index ≥30 kg/m2, current smoking, hypertension, diabetes, and total cholesterol ≥200 mg/dl.
Evaluation of vascular risk factors and late-life brain amyloid
In the overall sample, elevated midlife BMI was the only vascular risk factor with a statistically significant association with elevated late-life brain amyloid (OR 2.06 (95% 1.16, 3.65). Risk factor-amyloid relationships did not differ by race as indicated by non-significant interaction terms (p=0.42).
Cumulative number of vascular risk factors
Increasing number of vascular risk factors from midlife, but not late-life, was associated with elevated brain amyloid. Thirty-one percent of individuals with no vascular risk factors in midlife had elevated amyloid in late-life, compared to 61% of individuals with at least two vascular risk factors in midlife who had elevated amyloid in late life (difference in proportions 30.4%; 95% CI 16.4%–44.3%). When evaluated continuously, each additional midlife vascular risk factor was associated with an increased odds of elevated SUVR (OR 1.41, 95% CI 1.09-1.83); similar results were seen when number of risk factors was categorized, and each category was compared to having no risk factors (table 3). More risk factors were associated with higher odds of elevated SUVR, with strongest associations in midlife, and with decreasing ORs associated with increasing numbers of risk factors as they were considered at older ages (figure 1). Fewer individuals had no vascular risk factors in late-life, but amyloid levels were elevated in 37% of this group; amyloid positivity was more frequent among people with ≥2 vascular risk factors in late-life (Table 3; compared to no risk factors, difference in proportions 18.5%, 95% CI 1.1%–53.1%).
Table 3.
Adjusted odds ratios (95% confidence intervals) for the association of midlife and late-life number of vascular risk factors with elevated SUVR (global cortex SUVR>1.2) overall and stratified by APOE ε4 genotype, N=322.
| Overall (N=322) | 0 APOE ε4 Alleles (n=220) | 1 or 2 APOE ε4 Alleles (n=100) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. with SUVR>1.2/Total No. (%) | Adjusted OR (95% CI) | No. with SUVR>1.2/Total No. (%) | Adjusted OR (95% CI) | No. with SUVR>1.2/Total No. (%) | Adjusted OR (95% CI) | |
| Midlife (Visit 1, 1987-1989) | ||||||
| 0 Vascular Risk Factors | 20/65 (30.8) | 1 (Reference) | 14/47 (29.8) | 1 (Reference) | 6/18 (33.3) | 1 (Reference) |
| 1 Vascular Risk Factor | 62/123 (50.4) | 1.88 (0.95, 3.73) | 37/85 (43.5) | 1.36 (0.61, 3.05) | 25/38 (65.8) | 3.10 (0.84, 11.50) |
| ≥2 Vascular Risk Factors | 82/134 (61.2) | 2.88 (1.46, 5.69) | 45/90 (50.0) | 1.86 (0.83, 4.14) | 37/44 (84.1) | 9.15 (2.27, 36.89) |
| Late-life (Visit 5, 2011-2013) | ||||||
| 0 Vascular Risk Factors | 13/35 (37.1) | 1 (Reference) | 6/23 (26.1) | 1 (Reference) | 7/12 (58.3) | 1 (Reference) |
| 1 Vascular Risk Factor | 37/82 (45.1) | 1.02 (0.43, 2.43) | 16/50 (32.0) | 1.38 (0.43, 4.39) | 21/32 (65.6) | 0.56 (0.12, 2.67) |
| ≥2 Vascular Risk Factors | 114/205 (55.6) | 1.66 (0.75, 3.69) | 74/149 (49.7) | 2.21 (0.78, 6.26) | 40/56 (71.4) | 1.03 (0.25, 4.29) |
Models adjusted for age (at visit 5, 2011-2013), sex, race, education level, APOE ε4 genotype.
Vascular risk factors included: body mass index ≥30 kg/m2, current smoking, hypertension, diabetes, and total cholesterol ≥200 mg/dl.
Figure 1.
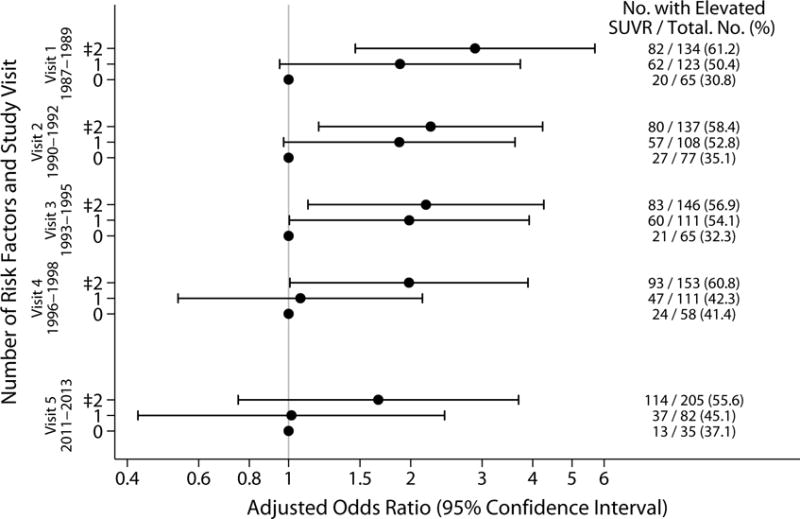
Adjusted odds ratios for elevated florbetapir by number of vascular risk factors, midlife through late-life.
Adjusted ORs and 95% CI are displayed for number of vascular risk factors for each visit, 1 through 5, for elevated SUVR (>1.2).
Models adjusted for age (at visit 5, 2011-2013), sex, race, education level, APOE ε4 genotype. Vascular risk factors included: body mass index ≥30 kg/m2, current smoking, hypertension, diabetes, and total cholesterol ≥200 mg/dl.
The observed association between number of risk factors and elevated odds of amyloid was only found in whites (supplemental table 2), with an increased odds of elevated amyloid per additional vascular risk factor in midlife (OR 1.66, 95% CI 1.15-2.39) in whites, compared to a smaller and nonsignificant increase in blacks per additional risk factor (OR 1.26, 95% CI 0.85-1.88). However, the statistical test for interaction of race by number of risk factors was not significant (p=0.42). Similarly, a significant association was found in men, but not in women, with imprecise estimates, and nonsignificant tests for interaction (supplemental table 3).
Association of APOE with vascular risk factor/brain amyloid associations
No interaction p-values for individual vascular risk factors by APOE status reached statistical significance. Odds of elevated florbetapir in participants with elevated BMI was statistically similar regardless of APOE status (OR 2.48, 95% CI 1.26-4.88 in APOE ε4 carriers and OR 1.11, 95% CI 0.85-1.44 in noncarriers). All of the 5 individuals with diabetes in midlife who were included in the study had elevated SUVR, but continuous fasting glucose from visit 1, in the 299 participants who fasted, was neither significantly associated with florbetapir uptake in people with an ε4 allele (OR 1.96, (95% 0.74-5.19) nor in those without (versus 0.89 (95% CI 0.61-1.32)).
When continuous SUVR was evaluated visually, increasing numbers of ε4 alleles did appear to be associated with higher florbetapir in the setting of increasing vascular risk (figure 2).
Figure 2.
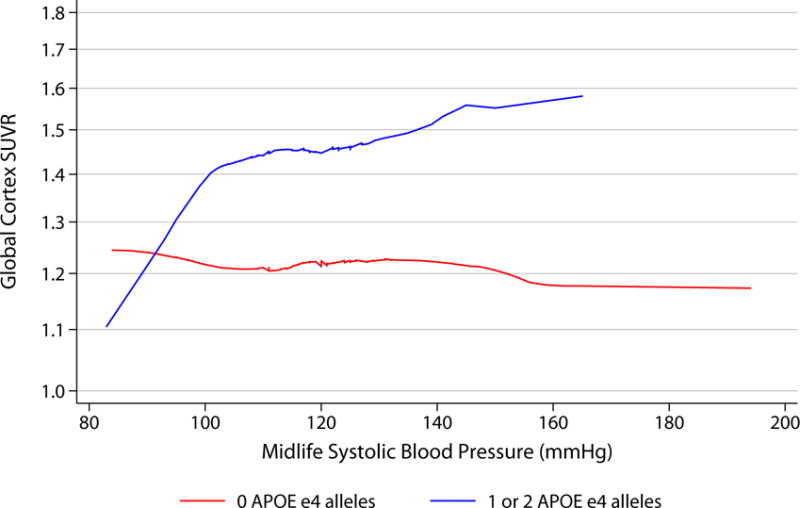
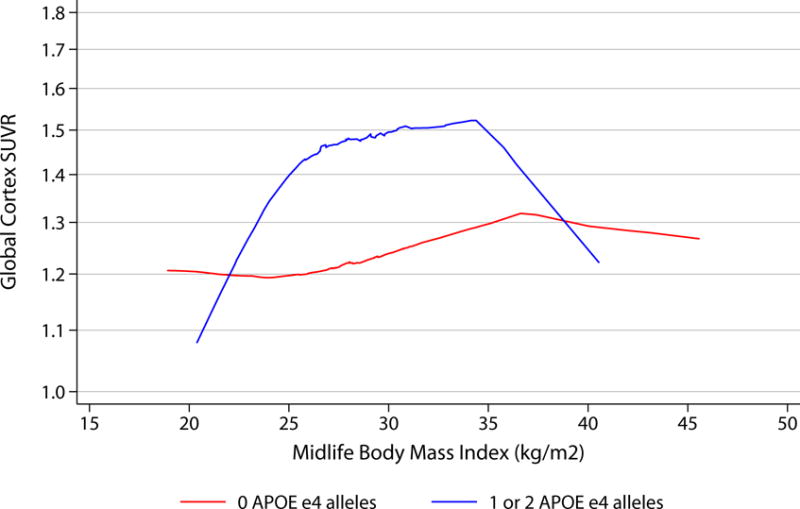
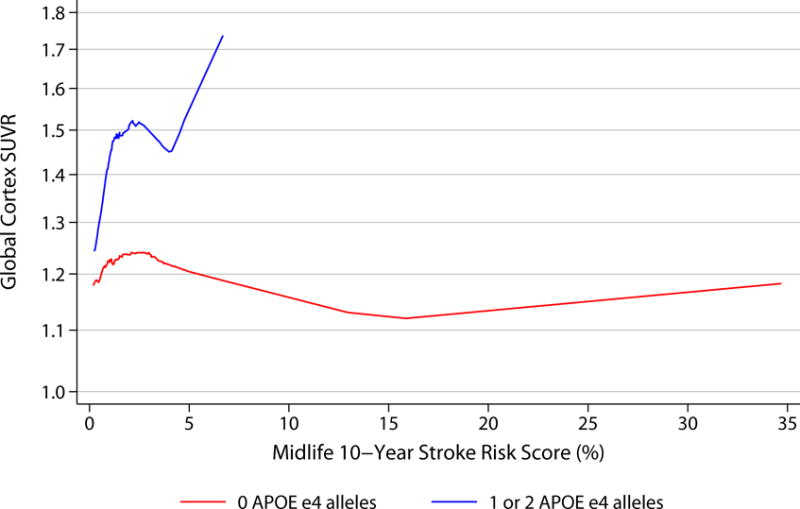
LOWESS curves demonstrating associations between midlife vascular risk factors and florbetapir by APOE status.
Curves show smoothed associations between vascular risk factors in midlife (visit 1, 1987-1989) and continuous global cortex Florbetapir SUVR, by APOE status (0 APOE ε4 alleles [n=222] versus 1 or 2 APOE ε4 alleles [n=100]).
Stratified analyses by APOE status showed no significant interaction between APOE and number of risk factors. There was no statistical difference in the odds of elevated amyloid in association with a larger number of midlife risk factors among APOE ε4 carriers (OR 2.46, 95% CI 1.37-4.42), versus noncarriers (OR 1.19, 95% CI 0.87-1.61), with similar results when number of risk factors was categorized (table 3). Increasing numbers of risk factors in late-life was not associated with increased amyloid, regardless of APOE status.
Sensitivity analysis
When analyses were repeated comparing individuals by cognitive status, estimates for individual risk factors were not statistically significant and were imprecise (supplemental table 4). Associations between number of vascular risk factors and amyloid positivity only remained statistically significant in the 87 individuals with MCI (supplemental table 5).
Results were similar with the SUVR cutpoints of 1.11 and 1.10 (supplemental table 6). Analysis of separate regions of interest yielded similar results to the primary analysis.
Imputing missing covariate data (for total N=343) resulted in very similar results for the risk factor analysis (OR for 1 midlife vascular risk factor, 1.81 (95% CI 0.92-3.56) and for 2 midlife vascular risk factors, 2.71, 95% CI 1.39-5.39, each compared to no midlife risk factors). Results were similar for evaluation of late-life vascular risk factor status when imputed data were included.
Discussion
In this study of brain florbetapir uptake in non-demented individuals from three U.S. communities, a cumulative number of midlife vascular risk factors was associated with elevated brain amyloid. Relationships between vascular risk factors and brain amyloid did not differ by race, despite previous findings in this study that amyloid distribution differs by race.14 Furthermore, these results were not supportive of a significant difference in association among people who were or were not carriers of an APOE ε4 allele. These data support the concept that midlife, but not late-life, exposure to these vascular risk factors, is more important for amyloid deposition.
Previous studies have demonstrated inconsistent results evaluating associations between vascular risk factors and brain amyloid. In multiple studies, diabetes has not been associated with elevated amyloid measured by Pittsburgh compound B (PiB) PET,24,25 whereas others have reported elevated amyloid in association with elevated vascular risk: elevated Framingham coronary risk score was associated with elevated PiB, independent of any APOE effect.26 Animal data do support a direct effect of vascular disease, especially hypertension, on the deposition of brain amyloid.27,28
The concept that vascular risk factors contribute to brain amyloid particularly, or even only, in the setting of an additional risk factor, namely APOE status, has been supported by previous studies. In this study, there was no statistical evidence for a difference by APOE status, but the study may have been underpowered to detect this interaction. In 118 cognitively normal adults, hypertension and APOE interacted in the prediction of risk of florbetapir amyloid uptake,29 and in an autopsy study, diabetes was only associated with AD neuropathology among APOE ε4 carriers.30 Further evaluation of these relative pathologies on cognitive performance is needed, since it is likely that vascular risk factors, APOE, AD neurodegeneration and amyloid deposition all play a role, some of which may affect each other, on cognition. The Framingham stroke risk score has been most strongly associated with cognition in individuals without increased AD neuropathology on autopsy,31 and in the Alzheimer’s Disease Neuroimaging Initiative, amyloid and vascular pathology acted additively and not synergistically, and independently of one another, increasing association with cognitive decline.32
This study focused on vascular risk factors, rather than clinical or subclinical vascular disease itself. Subclinical vascular disease might mediate the association of cumulative vascular risk factors with amyloid deposition. Although some authors have failed to find an association between radiographic vascular disease on MRI and PiB,33 arteriolar disease on autopsy has been associated with worsening AD neuropathology, independent of infarcts,34 suggesting that there may be aspects of brain vascular disease that, if adequately measured, might be important in increasing risk of amyloid deposition. An alternative hypothesis, supported by the findings of this study, is that vascular disease, particularly at the arteriolar level, might reduce vascular clearance of amyloid.35,36
This study has several limitations. The lack of an association with individual vascular risk factors may not reflect a true null relationship but instead may reflect inadequate sample size; the same may be true for the lack of an association in blacks (inadequate power to detect these associations or to detect interactions by race) or in noncarriers of an APOE ε4 allele. This relatively modest sample size makes it difficult to make conclusions about any true interaction by APOE status. By excluding individuals with dementia, it is likely that those ARIC participants seen in 1987-1989 with the highest amounts of both vascular disease and amyloid deposition were excluded from these analyses because they became demented or died prior to ARIC-PET. Thus, in reaching late life with relatively normal cognition, it might be expected that the opposite relationship would have been observed, namely that those ARIC survivors with multiple midlife risk factors would have had less brain amyloid. These APOE findings would argue against this concern about selection bias, however, since individuals with all three risks for dementia (APOE ε4 carriage, vascular risk, and elevated amyloid deposition) survived to participate in this study. Furthermore, analysis by cognitive status demonstrated that higher vascular risk in midlife was associated with elevated amyloid among individuals with MCI at the time of amyloid imaging.
Relatively few people with very high vascular risk in midlife survived to the PET visit, or met entry criteria for the study, supporting the likelihood that the true strength of association of vascular risk with amyloid risk may have been underestimated, as a result of this survival bias. Furthermore, although representative from the community, this study has higher prevalence of diabetes in late-life than has otherwise been described,37 with very high rates of hypertension; the frequency of these risk factors might dilute their association in late-life. In addition, the chronologic distinction between midlife and late-life is not sharp, so some participants at visits 2 and 3, when an association (although decreased) between number of risk factors and amyloid positivity was still noted, were in their 60’s. In addition, prevalence of amyloid positivity is reported to be under 20% in persons with normal cognition in the age range of our cohort,38 but without PET at the midlife visit we cannot be sure that none of our participants were amyloid positive at the time of their first visit.
Conclusions
An increasing number of midlife vascular risk factors was significantly associated with elevated amyloid SUVR; this association was not significant for late-life risk factors. These findings are consistent with a role of vascular disease in the development of Alzheimer’s Disease.
Supplementary Material
Table 2.
Adjusted odds ratios (95% confidence intervals) for the association of midlife and late-life vascular risk factors with elevated SUVR (global cortex SUVR>1.2) (N=322).
| Midlife vascular risk factor (Visit 1, 1987-1989) |
Late-life vascular risk factor (Visit 5, 2011-2013) |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. with Vascular Risk Factor and SUVR>1.2/Total No. with Vascular Risk Factor (%) | No. without Vascular Risk Factor and SUVR>1.2/Total No. without Vascular Risk Factor (%) | Adjusted OR (95% CI) | No. with Vascular Risk Factor and SUVR>1.2/Total No. with Vascular Risk Factor (%) | No. without Vascular Risk Factor and SUVR>1.2/Total No. without Vascular Risk Factor (%) | Adjusted OR (95% CI) | |
| Body mass index ≥30 kg/m2 | 54/83 (65.1) | 110/239 (46.0) | 2.06 (1.16, 3.65) | 66/121 (54.6) | 98/201 (48.8) | 1.44 (0.85, 2.44) |
| Current Smoking | 30/55 (54.6) | 134/267 (50.2) | 1.15 (0.61, 2.19) | 9/16 (56.3) | 155/306 (50.7) | 1.53 (0.50, 4.62) |
| Hypertension | 55/95 (57.9) | 109/227 (48.0) | 1.30 (0.75, 2.28) | 125/230 (54.4) | 39/92 (42.4) | 1.29 (0.74, 2.26) |
| Diabetes | 10/20 (50.0) | 154/302 (51.0) | 1.06 (0.39, 2.86) | 68/130 (52.3) | 96/192 (50.0) | 1.06 (0.65, 1.74) |
| Total cholesterol ≥200 mg/dl | 101/180 (56.1) | 63/142 (44.4) | 1.33 (0.82, 2.19) | 54/94 (57.5) | 110/228 (48.3) | 1.17 (0.67, 2.05) |
Models adjusted for age (at visit 5, 2011-2013), sex, race, education level, APOE ε4 genotype, body mass index, current smoking, hypertension, diabetes, and total cholesterol at the same visit.
KEY POINTS.
Question
Are midlife vascular risk factors associated with later-life brain amyloid deposition?
Findings
In a prospective cohort study of 346 members of the community-based ARIC-PET cohort without dementia, having 2 or more midlife vascular risk factors compared with none was significantly associated with elevated amyloid deposition in the brain (61.2% vs 30.8%). There was no significant association for late-life risk factors.
Meaning
These findings are consistent with a role of vascular disease in the development of Alzheimer’s disease.
Acknowledgments
Disclosures: Dr. Gottesman serves as Associate Editor for Neurology® and receives research support from the NIH. Dr. Knopman previously served as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRX Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer’s Disease Cooperative Study; and receives research support from the NIH. Dr. Mintz previously received an honorarium as a reader for Avid on florbetapir studies (through 2013). Dr. Wong has had a number of contracts with Avid and Lilly administered through Johns Hopkins University and has served as a consultant at the early stages of florbetapir development and has received and worked with this radiotracer as part of research collaborations including ADNI and Biocard (NIH). He is working with all 3 FDA approved amyloid PET radiopharmaceutical companies on the CMS/Medicare funded IDEAs trial.
Funding Source: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, HL096917 and the National Institute of Neurological Disorders and Stroke, with previous brain MRI examinations funded by R01-HL70825 (from the NHLBI). The ARIC-PET study is funded by the National Institute on Aging (R01AG040282). Avid Radiopharmaceuticals provided the florbetapir isotope for the study, but had no role in the study design or interpretation of results. Further, neither Avid nor the primary sponsor (NIA/NIH) had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors thank the staff and participants of the ARIC study for their important contributions. Thanks to Andrew Crabb, MS (Johns Hopkins University) for oversight of the central data transfer and research PACS; Mr. Crabb received effort from grant funding for his role in the study. Dr. Gottesman, as principal investigator, had full access to all the data in the study and takes respnsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Freitag MH, Peila R, Masaki K, et al. Midlife pulse pressure and incidence of dementia: the Honolulu-Asia Aging Study. Stroke. 2006;37(1):33–37. doi: 10.1161/01.STR.0000196941.58869.2d. [DOI] [PubMed] [Google Scholar]
- 2.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274(23):1846–1851. [PubMed] [Google Scholar]
- 3.Alonso A, Mosley TH, Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry. 2009;80(11):1194–1201. doi: 10.1136/jnnp.2009.176818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. British Medical Journal. 2001;322(7300):1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53(9):1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 6.Hernan MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008;19(3):448–450. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- 7.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 9.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews Neuroscience. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004;141(2):137–147. doi: 10.7326/0003-4819-141-2-200407200-00013. [DOI] [PubMed] [Google Scholar]
- 11.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 12.Knopman DS, Griswold ME, Lirette ST, et al. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities-neurocognitive study. Stroke. 2015;46(2):433–440. doi: 10.1161/STROKEAHA.114.007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) Alzheimer’s and Dementia: Diagnosis, Assessment, and Disease Monitoring. 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman RF, Schneider ALC, Zhou Y, et al. The ARIC-PET Amyloid Imaging Study: Brain Amyloid Differences by Age, Race, Sex, and APOE. Neurology. 2016;87(5):473–480. doi: 10.1212/WNL.0000000000002914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Joie R, Perrotin A, Barre L, et al. Region-specific hierarchy between atrophy, hypometabolism, and Beta-amyloid (AB) load in Alzheimer’s disease dementia. Journal of Neuroscience. 2012;32(46):16265–16273. doi: 10.1523/JNEUROSCI.2170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saint-Aubert L, Nemmi F, Peran P, et al. Comparison between PET template-based method and MRI-based method for cortical quantification of florbetapir (AV-45) uptake in vivo. Eur J Nucl Med Mol Imag. 2014;41(5):836–843. doi: 10.1007/s00259-013-2656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45. J Nucl Med. 2010;51(6):913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landau SM, Mintun MA, Joshi AD, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72(4):578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi AD, Pontecorvo MJ, Clark CM, et al. Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer’s disease and cognitively normal subjects. J Nucl Med. 2012;53(3):378–384. doi: 10.2967/jnumed.111.090340. [DOI] [PubMed] [Google Scholar]
- 20.Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of ischemic stroke risk in the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2004;160(3):259–269. doi: 10.1093/aje/kwh189. [DOI] [PubMed] [Google Scholar]
- 21.Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 1994;14(7):1098–1104. doi: 10.1161/01.atv.14.7.1098. [DOI] [PubMed] [Google Scholar]
- 22.Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29(6):1075–1080. [PubMed] [Google Scholar]
- 23.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts RO, Knopman DS, Cha RH, et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med. 2014;55(5):759–764. doi: 10.2967/jnumed.113.132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran C, Beare R, Phan TG, Bruce DG, Callisaya ML, Srikanth V. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology. 2015;85(13):1123–1130. doi: 10.1212/WNL.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed BR, Marchant NL, Jagust WJ, DeCarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging. 2012;33(9):1979–1987. doi: 10.1016/j.neurobiolaging.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cifuentes D, Poittevin M, Dere E, et al. Hypertension accelerates the progression of Alzheimer-like pathology in a mouse model of the disease. Hypertension. 2015;65(1):218–224. doi: 10.1161/HYPERTENSIONAHA.114.04139. [DOI] [PubMed] [Google Scholar]
- 28.Faraco G, Park L, Zhou P, et al. Hypertension enhances AB-induced neurovascular dysfunction, promotes B-secretase activity, and leads to amyloidogenic processing of APP. Journal of Cerebral Blood Flow and Metabolism. 2016;36(1):241–252. doi: 10.1038/jcbfm.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Diaz-Arrastia R, Park DC. Risk factors for beta-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurology. 2013;70(5):600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malek-Ahmadi M, Beach T, Obradov A, et al. Increased Alzheimer’s Disease Neuropathology is Associated with Type 2 Diabetes and APOE e4 Carrier Status. Current Alzheimer Research. 2013;10(6):654–659. doi: 10.2174/15672050113109990006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hohman TJ, Samuels LR, Liu D, et al. Stroke risk interacts with Alzheimer’s disease biomarkers no brain aging outcomes. Neurobiol Aging. 2015;36(9):2501–2508. doi: 10.1016/j.neurobiolaging.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain. 2015;138(Pt 3):761–771. doi: 10.1093/brain/awu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchant NL, Reed BR, Sanossian N, et al. The Aging Brain and Cognition. JAMA Neurology. 2013;70(4):488–495. doi: 10.1001/2013.jamaneurol.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez J, Honig L, Elkind MSV, et al. Brain arterial aging and its relationship to Alzheimer dementia. Neurology. 2016;86(16):1507–1515. doi: 10.1212/WNL.0000000000002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta A, Iadecola C. Impaired A-beta clearance: a potential link between atherosclerosis and Alzheimer’s Disease. Frontiers in Aging Neuroscience. 2015;7(115) doi: 10.3389/fnagi.2015.00115. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marin-Padilla M, Knopman DS. Developmental aspects of the intracerebral microvasculature and perivascular spaces: Insights into brain response to late-life diseases. J Neuropathol Exp Neurol. 2011;70(12):1060–1069. doi: 10.1097/NEN.0b013e31823ac627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the U.S., 1988-1994 and 1999-2010. Ann Intern Med. 2014;160(8):517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


